| 11 th Annual Pediatric Critical Care Colloquium |
| Session/Time | Pulmonary II/Thu, 2:00 - 4: 00 PM |
| Title | Multicenter, Randomized, Controlled Trial (RCT) of LiquiVent Partial Liquid Ventilation (PLV) in Pediatric ARDS |
| Author | B Fuhnnan, J Blumer, L Toro-Figueroa, L Hennan, P Cox, S Curtis, J Meliones, T Green and the LiquiVent Study Group |
| Affiliation | Children's Hospital of Buffalo, Buffalo, NY |
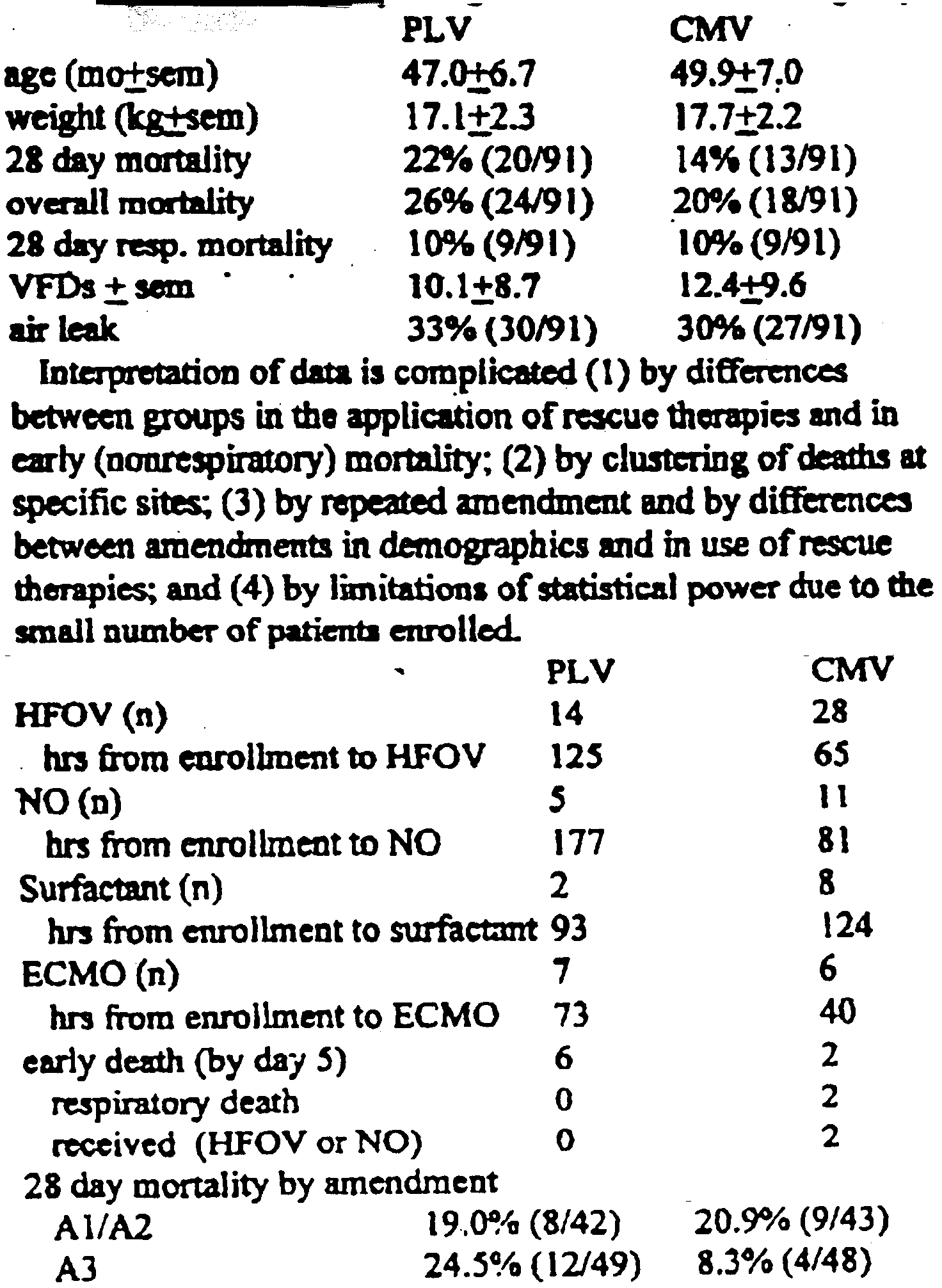
| Introduction | Between January 1996 and April 1997,182 pediatric patients wem emued at 65 sites in a Phase M'IU RCT of PLV using LiquiVenM (Alliance Pbarmaceutical Corp. APC). Patients were embed under thin separate amendments which mwifted entry criteria, use of rescue thepies and primary outmme endpoint Across amendments, enmflment was allowed for P2O2/FI02 <200 torr with bilaimi hifunam Endpohfts were 29. day and overall mdrwk, and venbla |
| Method | lnterpretation of data is COM lkmted (1) by diffences between groups in the application of rescue therapies and in early (oomspimory) mortality; (2) by clustering of deaths at specific sites (3) by repeated amendment and by differences betwem amendment in demogaphks and in use of rescue therapies, and (4) by limitation of statistical power due to the small number of patients enrolled. |
 |
|
| Though under-powered and terminated early, at a time when differences between groups appeared to warrant a safety check mortality was not different between groups. Findings support the safety of PLV with LiquiVent. |
Use your browser's back button to return to the appropriate index of abstracts...
Back to PCCC 98 Abstract Introduction | Back to PCCC 1998
Document created April 12, 1999