15th Annual Pediatric Critical Care Colloquium - Abstracts
Table of Contents
(published with permission from Pediatric Critical Care Medicine)
Bispectral Index Monitoring During the Administration of Neuromuscular Blocking Agents to Infants and Children in the Pediatric Intensive Care Unit
JD Tobias, MD
Departments of Anesthesiology & Pediatrics, University of Missouri, Columbia, Missouri
Introduction: Neuromuscular blocking agents (NMBA’s)
are occasionally required in the care of critically ill patients in the
PICU. One of the problems with
NMBA’s is that the clinical assessment of the depth of sedation is impossible.
Therefore, physiologic parameters (heart rate and blood pressure) may be used
to judge the depth of sedation.
The Bispectral Index (BIS Monitor, Aspect Medical, Newton, MA) uses a
specific algorithm to derive from the EEG a numerical value (0 to 100) of the
depth of sedation. Given the
inaccuracy of clinical sedation scores during the administration of NMBA’s and
the need to rely solely on physiologic parameters, we hypothesized that there
may be significant variations in the depth of sedation. The current study prospectively
evaluates BIS values during the use of NMBA’s and evaluates the accuracy of
decisions regarding the need for supplemental sedation.
Methods: This study was approved by the
hospital’s IRB and verbal consent obtained from a parent. Patients admitted to the PICU who
required the continuous infusion of a NMBA were included. Patients with a history of central
nervous system trauma or dysfunction, and mental retardation were excluded. During the use of the NMBA, all
patients were receiving a continuous infusion of midazolam or propofol with as
needed doses of midazolam, fentanyl, or morphine. The administration of supplemental sedation was at the
discretion of the nurse. The BIS
number was recorded by a computer every 10 seconds. The number from the BIS monitor was concealed from the nurse
and no clinical decision was based on the BIS number. Based on data from intraoperative clinical trials, a BIS
number of 50-70 was used as the desired level of sedation. Oversedation was defined as a BIS
number <50 and undersedation was defined as a BIS number greater
>70. Data are also presented
using a wider BIS range (40-70) as the desired level of sedation. The incidence of oversedation and
undersedation when using propofol versus midazolam was compared using a chi
square analysis with a Yates’ correction.
Results: Twelve patients (8 boys, 4 girls) ranging in age from 12 months to 12 years were studied. Data collection varied from 16 to 116 hours for a total of 476 hours (161,893 BIS values). Midazolam was used for 438 hours and propofol for 38 hours. The BIS number was within the desired range (50-70), 57% of the time; <50, 35% of the time, and >70, 8% of the time. With an expanded range of the desired level of sedation (BIS number 40-70), the BIS number was within this range, 63% of the time. There were 143 supplemental doses of sedation administered. When these supplemental doses were administered, the BIS number was >70, 64% of the time; 50-70, 31% of the time, and < 50, 5% of the time. Patients receiving propofol were more likely to have a BIS number <50 than patients receiving midazolam (p<0.0001).
Conclusions: During the use of NMBA’s, physiologic parameters are not an accurate means of judging the depth of sedation. The depth of sedation was appropriate to maintain the BIS at 50- 70, only 57% of the time. Thirty-six percent of the time when supplemental sedation was administered, the BIS number was <70. Given the potential for adverse effects with oversedation and the potential for awareness with undersedation, BIS monitoring may be helpful in guiding the depth of sedation during the use of NMBA’s.
PICU Sedation with Isoflurane and the “AnaConDa”
PV Sackey MD1 , C-R Martling MD PhD1, PJ Radell MD PhD2
1Depts. of Anesthesiology and Intensive Care, Karolinska Hospital and
Institute, and
2Pediatric Anesthesia and Intensive Care, Astrid Lindgren Children’s
Hospital.
Stockholm, Sweden
Introduction: Prolonged sedation in the PICU can be challenging. We report on initial experience with a new method to provide prolonged sedation with inhalational agents to difficult-to-sedate patients in the Pediatric ICU setting.
Methods: 3 male patients 4 to 12 years old and weighing between 20 and 40 kg received inhaled sedation for 6-8 days with isoflurane administered by the Anesthetic Conserving Device, AnaConDa® (ACD). Due to significant tolerance and inadequate sedation, two patients (Pat 1 and 3) with abdominal infections treated with open incisions were converted from intravenous sedation/analgesia and a third patient with refractory status epilepticus received therapeutic inhalational anesthesia with the ACD during continuous EEG monitoring. The ACD is an adapted heat and moisture exchanger which allows evaporation of liquid anesthetic agent given by standard syringe pump, eliminating the need for vaporizer and specially adapted ventilator. A carbon particle filter results in rebreathing of exhaled agent when the ACD is placed at the Y-piece of the respiratory circuit. The volume of the ACD is approximately 100 ml. Clinical adequacy of sedation or EEG activity was monitored and isoflurane administration titrated to effect. Isoflurane infusion rate, inspiratory and end-tidal isoflurane concentrations were monitored continuously. Hemodynamic, respiratory and renal function were monitored.
|
|
Pat 1 |
Pat 2 |
Pat 3 |
|
Age |
11y |
9y, 4mo |
4y, 1mo |
|
Weight, kg |
40,3 |
30 |
19,3 |
|
Hours of isoflurane
inhalation |
144 |
196 (98+98) |
187 |
|
Infusion rate, ml/hr.
(range) |
1,1 (1,0-2,2) |
2,2 (1,0-3,0) |
5 (3-6) |
|
Isoflurane inspiratory
conc., % (range) |
0,5 (0,3-0,8) |
1,1 (0,6-1,8) |
0,4 (0,2-0,4) |
|
Isoflurane end-tidal
conc., % (range) |
0,4 (0,3-0,45) |
0,9 ((0,5-1,4) |
0,3 (0,2-0,4) |
Results: Adequate sedation was achieved with end-tidal isoflurane concentrations of 0,3 to 0,4% while higher concentrations were needed to control seizure activity. Prolonged continuous inhalational sedation/anesthesia with isoflurane was provided without hemodynamic compromise. Analgesia with morphine and clonidine was continued at lower doses in the first two patients. Excessive dead space in the 20 kg patient (elevated end-tidal/arterial CO2, tachypnea) required moving the ACD from the Y-piece to the inspiratory limb of the breathing circuit, resulting in loss of the re-breathing function and higher isoflurane consumption. Clonus of the left foot was noted on day 5 in patient 1 and patient 3 had ataxic upper limb movements after extubation, both of which resolved within a few days after terminating isoflurane sedation. No tolerance was noted and no withdrawal symptoms were seen after termination.
Conclusions: This is the first report of the use of the AnaConDa to treat PICU patients with isoflurane. Inhalational sedation with the AnaConDa may provide an alternative method of sedation in difficult patients without the need for anesthesia equipment and warrants further study of efficacy, complications, cost-benefit and environmental health issues. To benefit from the re-breathing function of the AnaConDa, a smaller model is needed for patients under 30 kg.
Enteral vs. Parenteral Sedation/Analgesia in Intubated Infants with Bronchiolitis
A Torres, K Gumidyala, J Hamman, A Kesavan, M Knepp, J Kasap, K Skender, LR Evans
Department of Pediatrics, University of Illinois College of Medicine at Peoria, IL and the Children’s Hospital of Illinois at OSF St Francis Medical Center, Peoria, IL
Introduction: Problems typically encountered with continuous infusions of fentanyl and midazolam include need for intravenous access, quick development of tolerance, unpredictability of mental status recovery after discontinuation (especially midazolam), and expense. With the current approach to early enteral nutrition and the commercial availability of a well-tolerated enteral form of lorazepam, our approach to sedation and analgesia of the mechanically ventilated infant has evolved from continuous parenteral infusions of midazolam and fentanyl to intermittent scheduled doses of enteral lorazepam and methadone. The purpose of this study was to compare outcomes (i.e., medication charges, need for reintubation, complications) of intubated infants with bronchiolitis who received enteral methadone and lorazepam to the outcomes of those intubated infants who received intravenous midazolam and fentanyl.
Methods: The study was a retrospective chart review of all admissions to the PICU with bronchiolitis (ICD-9-CM code 466.11-466.19) requiring mechanical ventilation (96.70-96.72) from Fall 1999 to Spring 2002. Patient characteristics such as age, severity of illness, (PRISM II) and outcomes (e.g., hospital length of stay, time intubated, medication charges, complications, rate of reintubation) were collected. All continuous data (e.g., age, LOS) were compared for significant differences between the two groups using Mann Whitney U test. Proportions (e.g., reintubation rate) were compared for significant differences between two groups using chi square test. A p value < 0.05 was considered significant.
Results: There were 21 patients in the parenteral group and 14 patients in the enteral group. There were no significant differences in age, time intubated, hospital LOS, presence of comorbidities, concomitant infection or severity of illness between the two groups. Methadone and lorazepam were converted to fentanyl and midazolam equivalents, respectively for analysis. There were no significant differences in sedative and analgesics received or outcomes (see tables below). There was a significant difference in medication charges to the patients. The parenteral group received significantly more rocuronium than the enteral group (4.7 + 7.9 vs. 0.5 + 1.0 mg/kg per day, respectively; p=0.008).
|
Medications* |
Parenteral
Group |
Enteral Group |
p value |
|
Midazolam |
6.0 + 6.7 |
3.7 + 2.7 |
0.28 |
|
Fentanyl |
33.8 + 30.4 |
22.4 + 23.4 |
0.34 |
* Fentanyl reported as mcg/kg per day and midazolam
reported as mg/kg per day.
|
Outcome Variables |
Parenteral
Group |
Enteral Group |
p value |
|
Reintubations (%) |
19 |
14 |
1.0 |
|
Complications (%) |
33 |
29 |
1.0 |
|
Analgesic charges ($)* |
1.39 + 1.52 |
0.10 + 0.08 |
0.001 |
|
Sedative charges ($)* |
4.35 + 3.18 |
1.10 + 0.69 |
0.001 |
* Reported in 2004 US dollars for total mg/kg per day of
sedatives received and total mcg/kg per day of analgesics received.
Conclusions: Enteral sedation and analgesia with lorazepam and methadone are viable alternatives. A prospective study to assess other outcomes such as rate of withdrawal syndromes between the two routes is warranted.
Developmental Differences in Nutrient Transporter
Expression
in Developing Rat Myocardium
G Ofori-Amanfo, S Gangadharan, B Babic, CL Schleien, SJ Vannucci
Department of Pediatrics, Division of Pediatric Critical Care Medicine, Children’s Hospital of New York-Presbyterian, Columbia University College of Physicians and Surgeons, New York, NY
Introduction: The adult heart relies on free fatty acids as the major source of ATP under aerobic conditions. The neonatal heart, however, has limited capacity to utilize free fatty acids and relies predominantly on glucose for energy. The major transport proteins responsible for substrate transfer into the myocyte are the facilitative glucose transporter, GLUT, monocarboxylate transporter, MCT, and carnitine palmitoyltransferase, CPT. The unique nutrient transport capacity of the myocardium impact on substrate utilization. We hypothesize that myocardial expression of the transporters is under developmental modulation relative to the unique substrate requirements during development. This experiment investigates the expression of GLUT1, GLUT4, and MCT1 in developing myocardium to establish the time of switch of the nutrient preference and potential differences between the RV and LV.
Methods: 5 groups of rats were studied at postnatal ages 1, 7, 14, 21 days and adult. Following anesthesia, hearts were extracted and dissected into RV and LV. Total membranes were isolated from tissue homogenates and GLUT1, GLUT4 and MCT1 expression determined by western blot.
Results: There is a sharp decline in GLUT1 expression at postnatal day 7. By P14 GLUT1 expression has reached undetectable levels. At P7 the RV appears to retain more GLUT1 than the LV. GLUT4, the insulin senstive GLUT, expression is very low at birth, increases with age and peaks in adulthood. There is no interventricular difference in GLUT4 expression. MCT1 expression is high at birth and declines during adulthood when its expression is greater in the LV.
Conclusions: There
are differences in nutrient transporter expression in the developing myocardium
reflecting developmental differences in substrate requirement of the
myocardium. There are also differences between RV and LV. This experiment sets
the platform for studying the developmental differences in myocardial response
to ischemic stress and to investigate metabolic modulations that may be
beneficial in cardioprotection.
Pediatric Intensive Care Unit (PICU) Utilization and
Outcome
in a Solid Organ Transplant Population
GE McLaughlin, BG Gelman, JW Kuluz, M Nares, GP Cantwell, AG Tzakis, T Kato
Depts. of Pediatrics and Surgery, University of Miami/Jackson Memorial Hospital, Miami, FL
Introduction: In contrast to bone marrow transplant recipients and children with human immunodeficiency virus infection, indications for PICU readmission after solid organ transplantation are not well described. We sought to describe the reasons for readmission and vital status outcome by transplant type and indication for admission for a large cohort of pediatric abdominal transplant recipients.
Methods: Databases maintained by the Division of
Transplantation, Department of Surgery and the Division of Pediatric Critical
Care Medicine, Department of Pediatrics were cross referenced to identify all
patients age 18 years of age or younger who underwent a solid organ transplant
from August 1994 through December 2004. Patients were
classified as intestinal, liver, or kidney transplant recipients. Through
medical record review the indication for each PICU readmission was identified
as was the PICU outcome at discharge. Patients admitted for monitoring
of medication administration or for procedural sedation were excluded. Each
readmission was assigned to only one category. For example, if rejection was
thought to be the cause of bacteremia or hypovolemic shock, the readmission was
coded as rejection.
Results: The demographic characteristics of 341 transplant recipients are described in Table 1.
Table 1. Demographic characteristics of pediatric solid
organ recipients
|
Organ Type |
No. Patients |
No. transplants |
Median age at transplant (yr) |
Sex (%male) |
Race (%white) |
Ethnic Group (%Hispanic) |
|
Liver |
178 |
204 |
2.4 (22 d-17) |
47 |
75 |
44 |
|
Intestine |
95 |
109 |
1 (0.5-17) |
57 |
78 |
21 |
|
Kidney |
68 |
70 |
11 (1-17) |
41 |
74 |
53 |
As shown in Table 2, patients in the above cohort were readmitted to the PICU 308 times over the 10 year time period. Liver transplant recipients were readmitted 118 times (0.7 admissions/patient), intestinal recipients were readmitted 168 times (1.8 admissions/patient) while kidney recipients were readmitted 22 times (0.3 admissions/patient).
|
Table 2. PICU readmission, indication, PRISM, length
of stay (LOS) and outcome |
|||||
|
|
Indication |
Number of
admissions |
PRISM |
LOS |
Survival |
|
Liver |
Surgical Complication |
28 |
11 |
11 |
100 |
|
|
Rejection |
10 |
5 |
3 |
87 |
|
|
Respiratory Failure |
12 |
9 |
22 |
58 |
|
Intestine |
Surgical Complication |
9 |
5 |
8 |
77 |
|
|
Rejection |
30 |
6 |
9 |
87 |
|
|
Respiratory Failure |
23 |
12 |
16 |
75 |
|
Kidney |
Surgical Complication |
5 |
9 |
6 |
100 |
|
|
Rejection |
4 |
19 |
31 |
75 |
|
|
Respiratory Failure |
2 |
6 |
6 |
100 |
Conclusions: Pediatric intestinal transplant recipients are younger and use more PICU resources with greater PICU mortality than liver or kidney recipients. Survival following respiratory failure appears to be greater than that previously described for pediatric bone marrow transplant recipients.
Implementing Innovative Solutions for PICU Expansion
in An Era of Critical Care Workforce Shortage
RC Sachdeva MD PhD and TB Rice M.D.
Department of Pediatrics, Medical College of Wisconsin, and National Outcomes Center, Children's Hospital and Health System, Milwaukee, WI (Supported, in part, by the Department of Management Sciences, University of Strathclyde, Glasgow, U.K.)
Introduction: Many Pediatric Intensive Care Units (PICUs) in the U.S. are in the process of expanding bed capacity. Previous studies have shown that staffing shortages, including nursing shortage, can lead to adverse patient outcomes. In the current environment of workforce shortage, ensuring an optimal level of staffing can be a significant challenge for most PICUs. Therefore, it is important to understand the inter-relationship of critical care physician and nurse staffing patterns on PICU efficiency and outcomes, and to explore innovative staffing solutions that will ensure optimal quality of care while expanding the number of PICU beds in an environment with staffing constraints.
Methods: Aims of this study – 1) to identify the impact and inter-relationship of physician and nurse staffing on PICU efficiency; 2) to identify the factors outside the PICU in other clinical settings at the Children's Hospital of Wisconsin (CHW) that impact PICU efficiency; and 3) to develop innovative solutions using simulation models to test the impact of various policy scenarios of expansion of numbers of PICU beds with varying numbers of staff, on PICU efficiency. This study was conducted at the CHW during 2001-2003 and consisted of three phases. Phase I: Historical data of all patients admitted to the PICU during the peak census months (representing the winter months with the highest patient census) were collected and analyzed to identify bottlenecks in patient flow and predictors of PICU efficiency. Data on severity of illness using the Pediatric Risk of Mortality (PRISM) score, and outcomes were collected on all patients. Phase II: Simulation models were developed to model the patient flow into and out of the PICU. Simulation methodology represents a relatively new analytic approach in healthcare and has been successfully used in many non-health care sectors to improve efficiency. The use of these simulation models allowed quantifying the impact of physician and nurse staffing and bed capacity, on patient flow, efficiency, and outcomes. Severity adjustment using PRISM scoring system was performed to further enhance the clinical applicability of the simulation models. Phase III: PICU expansion policies were developed based upon the interaction between physician and nurse staffing to optimize efficiency and quality of care.
Results: 397 consecutive admissions with medical and surgical diagnosis admitted to the PICU were included; Age=5.3± 6.3 yrs.; PRISM score=5.2±9.9. There is an inter-relationship between physician and nurse staffing in the PICU which impacts efficiency (p<0.05). Staffing in other parts of CHW outside the PICU also impacts PICU efficiency (p<0.05). Three bottlenecks for patient flow that impact PICU efficiency were identified. Predictors of these bottlenecks were identified using multivariate regression analysis (p<0.05). These predictors were used as parameters in the simulation models. Results from these simulation models were successfully used to develop new staffing policies, creating organizational budgets, and for strategic planning.
Conclusions: 1) Critical care physician and nurse staffing numbers impacts PICU efficiency. 2) There is an inter-relationship between physician and nurse staffing which also impacts PICU efficiency. 3) It is imperative to satisfactorily address staffing issues prior to bed capacity expansion to ensure no compromise in quality and outcome of patient care.
Application of Pediatric Critical Care Quality
Indicators
Using a PICU Clinical Database
MC Scanlon1, RC Wetzel2, TB Rice1
1Medical College of Wisconsin, Children’s
Hospital of Wisconsin
2 Children’s Hospital Los Angeles, University of Southern California
Background: Quality indicators have gained increasing attention and importance in the last decade. National guidelines for quality indicators recommend that measures be valid, relevant to the population considered, clearly defined, feasible to collect using valid data sets, and severity adjusted, when appropriate. Existing adult quality measures have limited applicability in a pediatric intensive care unit (PICU) setting and administrative data sets have limited utility. This abstract reports the application of pediatric critical care quality indicators to an existing PICU clinical database.
Methods: The 2002 NACHRI (National Association of Children’s Hospitals and Related Institutions) PICU Focus Group identified eight quality indicators for use in the PICU setting. Indicators were selected from existing adult measures and literature, using both existing national guidelines and a consensus opinion. These indicators reflected mortality, length of stay (LOS), LOS > 7 days, unscheduled readmissions within 48 hours, reintubation within 24 hours, delayed PICU discharge, and ventilator and noninvasive ventilator utilization.
The Virtual PICU Performance System© (VPS) (a joint product of the NACHRI and the Virtual PICU) was developed in 1999 to provide a comprehensive clinical dataset specific for pediatric critical care. Since its inception, 38 hospitals have contributed over 25,000 PICU cases to the VPS© database. For this project, blinded data from 18 PICUs was abstracted and compared based on the eight quality indicators. Additional comparisons were made between a single PICU and the remaining aggregate data set.
Results: Analysis of the data revealed significant variation in the quality indicators among PICUs and within a PICU over time. Divergence between the mean and median values for quality indicators reflects the non-parametric nature of the data. Comparison of the quality indicators when calculated with both total PICU discharges and individual patient PICU discharges in the denominator revealed differences within LOS.
Conclusions: We successfully applied quality indicators to a clinical PICU database and demonstrated the feasibility of these indicators to assess and potentially improve ICU care. Discrepancies in the mean and median values for quality indicators reinforce the importance of appropriate statistical analyses of quality indicator data, especially if the intended use is public reporting. Recognition of differences based on denominator selection reveals how practice variation and readmission influences quality measurement.
National Online Survey of Rule of Six versus Standardized Concentrations
MI Gaffoor MD, E Hilmas PharmD, L Mathews RN, W Morrison MD, and VU Vaidya MD
Division of Pediatric Critical Care, University of Maryland School of Medicine, Baltimore, MD
Introduction: There has been a significant ongoing debate among pediatric and neonatal critical care providers regarding the method of choice for ordering continuous infusions; the rule of six (ROS) versus standardized concentrations (SC) method. The debate has intensified since the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) mandated that all institutions use the SC method by January 2005. To-date there are no studies comparing the two methods. We conducted a national online survey to assess provider experiences and opinions comparing the two methods.
Method: A 60 question web-based survey was posted on multiple internet listservs aimed at pediatric and neonatal critical care providers. A total of 1483 responses were received of which 1150 were complete and used in the data analysis. Data analysis was performed using logistic regression except where otherwise noted to control for confounding factors.
Results: Our sample consisted of 494 (43%) nurses, 406 (35%) physicians, 155 (14%) pharmacists, and 81 (7%) nurse practitioners with the majority (60%) having greater than 10 years of clinical experience. Surprisingly, 32% of respondents were not aware of the JCAHO mandate. Of our sample, 59% of respondents are currently using ROS while 41% are using SC. Most respondents preferred the method they were currently using with 53% preferring ROS and 42% preferring SC (OR:28, p<0.001, 95% CI: 18.0-41.4)
When looking at respondents opinions regarding the strengths and weaknesses of SC and ROS we found their results to be dichotomized based on their overall preference of method. For example, 85% of those who prefer ROS disagree with the JCAHO mandate while only 10% of those who prefer SC disagree with the JCAHO mandate (p<0.001). Regarding respondents opinion about the loss of intuitive relationship between dose and infusion rate when using the SC method, 85% of ROS users feel that the lack of this relationship will lead to more nursing errors if SC method is used as opposed to 15% of SC users (p<0.001). This statistically significant trend was seen in almost every other question of opinion such as applicability of SC in the NICU as well as ease of use and safety of both methods for physicians, nurses and pharmacists.
We also noted that more respondents recalled sentinel (adverse) events with SC (67%) than with ROS (51%), (p<0.001 via two-sample test of proportion). However, respondents who preferred rule of six were more likely to report sentinel events with SC (OR: 1.9, p<0.001, 95% CI: 1.3-2.8) and respondents who preferred SC were more likely to report sentinel events with ROS (OR: 2.0, p<0.001, 95%CI: 1.4-2.9). A reanalysis was performed only looking at users’ recollection of sentinel events for their preferred method. Even when controlling for user bias in this fashion we noted that 64% recalled sentinel events with SC while 45% recalled sentinel events with ROS (p<0.001 via two-sample test of proportion)
Conclusions: Respondent’s opinions about the strengths and weaknesses of both methods were heavily weighted based on their individual preference for a particular method. In addition, despite this bias, respondents do recall more adverse events related to SC than to RO6. Based on this data, from a group of multidisciplinary and experienced respondents, and the lack of true scientific studies, it appears that we cannot categorically identify one method as being superior and safer than the other. A mandate to use only one method may be premature at this time.
Effect of Nitric Oxide on Leukocyte Adhesion to Astrocytes After Trauma
VA Uduaghan, B Wu, D He, J Kuluz
Department of Pediatrics, University of Miami School of Medicine, Miami, Florida
Introduction: Inflammatory mechanisms play an important role in secondary brain injury following ischemia and trauma. We developed an in vitro model of traumatic brain injury, induced by physical disruption of a monolayer of primary astrocytes in culture using a plastic pipette tip. We advanced this model to include cellular inflammation by co-culturing leukocytes obtained from whole blood from donor animal. In previous studies we have shown that following trauma, injured astrocytes adjacent to the injury express cellular adhesion molecules and that leukocytes adhered preferentially along the edge of the trauma. The purpose of this study was to determine the role of nitric oxide (NO) in the interaction between leukocytes and injured astrocytes. We hypothesized that leukocytes adhere to traumatized astrocytes in culture and that this adhesion is inhibited by NO and promoted by NO synthase inhibition.
Methods: Primary astrocyte cultures were prepared from cerebral cortex of one-day-old rat pups. Cultures that were at least 6 weeks old and had reached confluence were used. An in vitro model of post-traumatic neuroinflammation was created by co-culturing leukocytes (obtained from whole blood) with astrocytes following trauma. The traumatic injury was produced by scratching confluent astrocytes with a 200µl sterile plastic pipette tip, according to a predetermined grid. Different concentrations of L-arginine (substrate for NO production; L-Arg; 0.5mM to 2mM), sodium nitroprusside (NO donor; SNP; 10 to 100µM) and 7-nitroindazole (NO synthase inhibitor; 7-NI; 10 to 100µM) were added to astrocyte cultures 16 hours after injury. Leukocytes were labeled with a fluorescent marker, added to astrocytes 18 hours after injury and co-cultured for 30 minutes, at which time non-adherent leukocytes were washed away with phosphate buffer solution (PBS). Leukocyte adhesion (expressed as per cent of baseline) was quantified by counting the total number of adherent leukocytes in 10 hpf under fluorescent microscopy.
Results: 7-NI, at concentrations of 50µM and 100µM, significantly increased the number of leukocytes that adhered to injured astrocytes (p<005). As shown in the graph below, L-Arg and SNP significantly decreased the number of adherent leukocytes (p<0.05). These effects were concentration dependent.
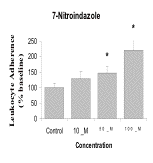
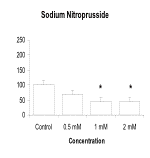
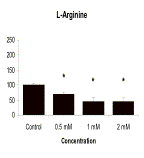
Conclusions: Leukocyte adherence to injured astrocytes in culture is reduced by the NO precursor, L-Arg, and by the NO donor, SNP, but increased by the NO synthase inhibitor, 7-NI, all in a concentration dependent manner. This suggests that NO may reduce post-traumatic neuroinflammation by reducing adhesion of leukocytes to astrocytes.
Cerebral Tissue Oxygen Saturation (PbtO2) in
Pediatrics—
Experience with the Licox Catheter in 13 Patients
G Goodman, S Palmer, and MK Bader
Children’s Hospital of Orange County (CHOC) at Mission / Mission Hospital
Mission Viejo, California
Introduction
PbtO2 has been increasingly recognized as a valuable measurement in severe traumatic brain injury (TBI). We implanted Licox (Integra Life Sciences) catheters in the brain in 13 children. We discuss the implantation procedure, the clinical utility, and the complications.
Materials and Methods
The 13 patients ranged in age from 3 months to 17 years. There were 11 males and 2 females. GCS scores averaged 6.7 on admission and 5.2 after resuscitation and admission to the pediatric ICU. ISS scores averaged 24.8. All catheters were placed in the operating room in tandem with temperature probes and ventriculostomies. All patients were treated according to a severe TBI protocol specifically modified for children.
Results
Patients were monitored an average of 10 days. Mechanical ventilation averaged 12.8
days. ICU stays averaged 15 days and hospital stays averaged 19.5 days. GOS scores, measured at 6 months post
injury, were 1 in 2 patients, 3 in 1 patient, 4 in 3 patients, and 5 in 7
patients (avg 4.1). There were no
monitor related hemorrhages, infections, or other complications. Ischemia
(PbtO2 levels < 20 mm Hg) was seen in the first 24 hours in 80% of the
patients and hyperemia, PbtO2 > 30mm Hg, was common in the majority of cases
after 24 hours, both of which could be clinically controlled with
manipulation of FIO2, CO2, MAP, ICP, and blood product administration.
Conclusions
The PbtO2 was highly sensitive to changes in FIO2, pCO2, CPP, ICP, HCT, and sedative usage. The catheters can be implanted without significant morbidity or mortality. Monitoring PbtO2 allows effective treatment of cerebral hyperemia and ischemia leading to improved patient outcomes in pediatric severe TBI.
Hypoglycemia in Immature Brain Has Long-Lasting
Neuropathologic
And Behavioral Consequences
LM Grimaldi MD1, B Babic BA1, S Brunelli PhD2,3, H Moore PhD2, SJ Vannucci PhD1
1Department of Pediatrics, Division of Pediatric Critical Care Medicine, Columbia University College of Physicians and Surgeons, Children’s Hospital of New York Presbyterian, New York, NY; 2Department of Psychiatry; 3New York State Psychiatric Institute, Department of Developmental Psychobiology
Introduction: Hypoglycemia is the most common metabolic derangement encountered in infants and children. Young children with insulin-dependent diabetes mellitus (IDDM) are particularly susceptible to recurrent bouts of hypoglycemia during the critical period of brain development. The effects of hypoglycemia on neurologic development are unknown, but a multitude of clinical studies have reported long term cognitive and behavioral abnormalities in childhood-onset IDDM, suggestive of permanent neurologic damage. In this study, a model of repetitive insulin-induced hypoglycemia in the immature rat was used to investigate the neurologic and psychopathological consequences.
Methods: Wistar rat pups of both genders were cross-fostered at P1 to avoid prenatally-determined litter effects. Pups were injected twice daily with saline or insulin 5U/Kg s.c. in 0.1cc from post-natal day (P)9 - P22. Pups were returned immediately to the dam. A subset of pups was tested for maternal separation-induced ultrasonic vocalization (USV) at P14, acoustic startle response at ages P19, P30 and P40-42, and play behaviors on P 33-35. In a separate set of pups, brains were processed for in situ end labeling (ISEL), immunohistochemistry or mRNA quantification.
Results: At P16, P26 and P35, increases in GFAP and GLUT5 mRNA, indicative of astogliosis and microgliosis, respectively, were observed in the dentate gyrus of the hippocampus, the hypothalamus (arcuate and paraventricular nuclei) and median eminence accompanied by DNA fragmentation. Behaviorally, hypoglycemic pups exhibited increased maternal separation-induced USV at P14 and decreased habituation to startle on P19 and P26. Moreover on P33-35, more than 12 days following the last insulin treatment, the insulin-treated group showed a play deficit as indicated by a decrease in a composite score quantifying walk-overs, napes, and pins. There were no apparent differences in non-play behaviors such as self-grooming and rearing.
Conclusions: Recurrent hypoglycemia in the immature rat has clear neuropathologic and behavioral consequences which are manifested early and persist long after the return to normoglycemia. The behavioral abnormalities demonstrated by the insulin-treated animals correlate with the neuropathologic changes seen in the hippocampus and hypothalamus. These results call in to question the safety of tight glucose control in juvenile diabetics during this crucial period in brain development.
Regulation of Developing Neurons by Receptor Protein
Tyrosine Phosphatases
MR Gonzalez-Brito DO and JL Bixby PhD
Department of Pediatric Critical Care Medicine and
The Miami Project to Cure Paralysis, University of Miami/Jackson Memorial Hospital,
Miami, FL
Introduction: Neurotrauma in the form of traumatic
brain injury (TBI) and spinal cord injury (SCI) present a significant challenge
to critical care physicians. After
injury, the brain and spinal cord neurons fail to extend their axons and do not
grow. Molecules which regulate
nerve growth and axon guidance may stimulate the regeneration of damaged nerves
and provide a treatment for TBI and SCI.
Receptor Protein Tyrosine Phosphatases (PTPs) are a family of proteins
which regulate nerve outgrowth and axon guidance during development. Requirements for neuronal development
include adhesion, outgrowth/extension of the axon, and proper guidance of the
axon to reach its target. We
describe how the delta, RO, and leukocyte common antigen-related (LAR) PTPs
regulate these functions during neuronal development in vitro and in vivo.
Methods: 1. In vitro Outgrowth assays: A truncated PTP-delta fusion protein, resembling the isoform that predominates during development, was constructed and expressed in CHO cells and tested in assays for adhesion and neurite outgrowth. For these assays, E7 Chick forebrain neurons were plated on substrates of various proteins. Neurons were incubated for 2 hrs (cell adhesion assay) and 16 hrs (neurite growth assay) prior to washing and fixation. 2. In vivo Neuronal Growth: Single RO and double RO/LAR mutant mice were perfused and fixed at P0 to collect lumbar spinal cord. The tissue was processed, paraffin-embedded, H&E stained, and sectioned on a microtome at 10μm thickness. Finally, total L4 DRG neuronal counts were performed using a stereology microscope and Stereo Investigator software. 3. In vivo Axon Guidance: DiI was placed along the lumbar spinal column of perfused and fixed P0 mutant and wildtype mice. Next, the samples were incubated at 37°C for 2 weeks and then sectioned on a vibratome at 200μm thickness.
Results: 1. The truncated delta isoform was less effective for adhesion and more potent in neurite promotion than the full-length isoform. 2. RO knockout mice have no decrease in total DRG neuronal survival and modest central afferent guidance errors. 3. RO/LAR knockout mice display more severe central afferent guidance errors than the RO mutant animals, suggesting these proteins cooperate to regulate axon guidance.
Conclusions: The delta, RO, and LAR PTPs regulate developing nerve outgrowth and axon guidance in vitro and in vivo. Specifically, the delta isoform that predominates during development promotes more neurite outgrowth than the full-length. The results suggest that different regions of the delta extracellular domain (ECD) may be responsible for these differences in function. Preliminary data on the RO mouse knockouts suggest a minority of the DRG neurons may require RO for survival and guidance, and these guidance errors are amplified by removing the LAR gene. This suggests that RO may regulate a sub set of DRG neurons by cooperating with LAR. Future studies examining differential sensory functions and populations of DRG neurons in these animals are needed to confirm these findings.
Circadian Response of Pro-Inflammatory Cytokines
to Lipopolysaccharide Injection in Mice
G Malkani, M Meyer, C Katyal, HM Ushay, BM Greenwald, ZS Sun
Pediatric Critical Care Medicine, Weill Medical College of Cornell University, New York, NY
Introduction: Circadian rhythms are the daily oscillations of multiple biological processes driven by endogenous clocks. Lipopolysaccharide (LPS), a major component of the outer membrane of gram-negative (GN) bacteria, is the endotoxin responsible for initiation of the host response. We have previously shown a circadian variation in mortality, in mice injected intraperitoneally (IP) with E.coli LPS. Mice injected with LPS at 9am demonstrated a 0% mortality rate versus 40% at 2pm. We hypothesize that the circadian clock system plays a role in innate immune responses to LPS injection in mice.
Methods: Two groups of C57BL6 mice were injected IP with E.coli LPS (25mg/kg). Group 1 at ZT3 (9am) (Zeitgeber Time) and Group 2 at ZT8 (2pm). The time points were chosen based on the greatest difference in mortality on the mouse survival curve. Mice were sacrificed (n=5) at predetermined intervals after the injections, and plasma obtained by intracardiac puncture. Pro-inflammatory cytokine levels (IFN-g , TNF-a , IL-6 and IL-1b ) were measured by ELISA.
Results: All four pro-inflammatory cytokines showed a significantly greater response to the LPS injection at 2pm, as compared to 9am (please refer to table). This correlated with higher mouse mortality rates in response to LPS at 2pm (40%) compared to 9am (0%).
Conclusions: We conclude that:
1. The pro-inflammatory cytokines (IFN-g , TNF-a , IL-6 and IL-1b ) demonstrated a circadian responsiveness.
2. Higher pro-inflammatory cytokine levels were associated with greater mortality rates in mice injected with LPS.
3. The innate immune response to GN endotoxin in mice is modulated by the circadian clock system.
|
Cytokine |
Cytokine Level |
Cytokine Level |
P Value |
|
IFN-g at T9 |
10018.1 |
20965.8 |
0.003 |
|
TNF-a at T1 |
2238.39 |
4356.32 |
0.0038 |
|
IL-6 at T9 |
151686.5 |
397482 |
0.0167 |
|
IL-1b at T9 |
186.705 |
1007.72 |
0.0085 |
T1 = 1 hr after LPS injection
T9 = 9 hr after LPS injection
Microalbuminuria Levels Are Correlated with PELOD Scores in Critically Ill Children
MK Wakeham MD, KL Rajzer RN, DB Angst DNSc, LE Torero MD,
DG Jaimovich MD
Pediatrics, Hope Children’s Hospital, Oak Lawn, IL
Introduction
Microalbuminuria (MA), a sub-clinical increase in urinary albumin, is a recognized marker of systemic inflammation, and is thought to reflect the glomerular component of a systemic capillary leak. Previous research has shown that sustained MA is associated with the development of later organ dysfunction and poor outcomes in adults. To date, the relationship of MA and organ system dysfunction (OSD) in critically ill children has not been systematically evaluated. The purpose of this study was to examine the relationship between MA and OSD in critically ill children.
Methods
Eligible subjects were patients < 18 years, who were admitted to the PICU, and anticipated to stay >24 hrs. Patients with primary nephropathies or gross hematuria were excluded. Microalbuminuria creatinine ratios (MACR) were obtained from each patient at admission (MACR1) and at 24 hrs (MACR2), and expressed in mcg of albumin per mg of creatinine. Daily PELOD scores were calculated for each patient. Correlations between a patient’s highest MACR and PELOD scores were performed. In addition, differences in highest MACR between patients with and without multi-organ system dysfunction (MOSD) were examined using the Student’s t test. A p<0.05 was considered statistically significant.
Results
The sample included 41 patients, 24 medical, and 17 surgical with a median age of 29 months (range 1 to 189), and median PRISM score of 12 (range 2 to 39). Four (9.8%) patients had no OSD, 16 (39%) had 1 OSD, and 21(51.2%) had MOSD. MACR was significantly correlated with the PELOD score on day 1(r = 0.85, p<0.0001). Patients with MOSD had a significantly higher MACR, than patients without MOSD (1115.79 +/- 944.52 vs.131.05 +/- 223.30 respectively, p<0.0001). Twelve patients had increasing MACR during their first 24 hrs (MACR2>MACR1). An increasing MACR had 75% PPV and 100% NPV for a higher PELOD score on days 2 or 3.
Conclusions
This study demonstrates a significant correlation between microalbuminuria and the degree of organ system dysfunction in critically ill children. It also suggests that rising microalbuminuria is predictive of worsening organ dysfunction. Microalbuminuria can be rapidly determined, is inexpensive, blood sparing, and it may have a role in the clinical assessment of the critically ill child.
Active Compression-Decompression Plus Inspiratory Impedance Threshold Device CPR Results in More Efficient Cold Transfer between Blood and Brain Than Standard CPR During Cardiac Arrest
V Srinivasan1, V Nadkarni1, D Yannopoulos2, S McKnite2, B Marino1, G Sigurdsson2,
D Benditt2, M Helfaer1, K Lurie2,3
1Dept of Anes/CCM, CHOP, Phila, PA; 2Card Arrhythmia & Crit Care Ctr, 3Depts of Int Med & Emerg Med, U of Minn, Minneapolis, MN
Introduction: Active Compression-Decompression cardiopulmonary resuscitation with inspiratory Impedance Threshold Device (ACD-ITD CPR) is superior to standard CPR (S-CPR) for rapid induction of cerebral hypothermia for neuroprotection during cardiac arrest (CA). We hypothesized that body temperature gradients (ΔT) measured during hypothermia induction during and following cardiac arrest are smaller in ACD-ITD CPR vs. S-CPR with more efficient cold transfer between blood and brain.
Methods: 16 propofol anesthetized pigs with ventricular fibrillation induced x 8 min without intervention were randomized to receive either ACD-ITD CPR (n=8) or S-CPR (n=8). After 2 min of CPR, 30mL/kg 0oC iced 0.9% saline was infused over the next 3 min of CPR via central femoral vein followed by defibrillation attempts (150J, biphasic) and ACLS resuscitation protocol until ROSC for 15 min or death. Temperatures (oC) were recorded at the brain (2cm depth in cortex), and ascending aorta. ΔT between blood and brain compartments was compared between ACD-ITD CPR and S-CPR by t test.
Results: Arterial blood temperature decreased similarly in both ACD-ITD CPR (37.9 + 0.4 to 32.5 + 2 °C) and S-CPR (37.9 + 0.5 to 32.1 + 1.6 °C, p=0.6) during infusion of ice-cold saline. At 1 min of ROSC, the brain-blood ΔT was significantly smaller in ACD-ITD CPR (-1.6 + 1.5 °C) vs. S-CPR (-5.2 + 1.7 °C, p<0.01). By 5 min of ROSC and beyond, the differences in brain-blood ΔT between ACD-ITD CPR and S-CPR were no longer significant.
p<0.01
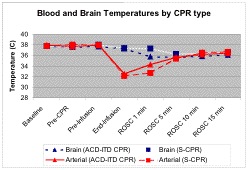
Conclusions:
Blood-brain temperature gradients measured during hypothermia induction immediately
following return of spontaneous circulation after cardiac arrest are smaller in
ACD-ITD CPR compared with standard CPR, with more efficient cold transfer
between blood and brain compartments. This study suggests that ACD-ITD CPR
would be more efficient than standard CPR at inducing brain cooling especially
if CPR were prolonged with continued induction of hypothermia. (Supported by
Endowed Chair, CCM, CHOP and Card Arrythmia Ctr, U Minn)
Preliminary Results of The Effects of Oral Arginine on Exhaled Nitric Oxide Concentrations in Sickle Cell Patients with History of Acute Chest Syndrome
KJ Sullivan, N Kissoon, E Sandler, J Sylvester, J Lima, L Duckworth, M Froyen, SP Murphy
Departments of Pediatric Anesthesiology and Critical Care Medicine, Nemours Children’s Clinic & the University of Florida, Jacksonville, FL
Introduction: Aberrations in the production and metabolism of nitric oxide (NO) have been implicated in the pathogenesis of sickle cell anemia (SCA). During baseline health exhaled nitric oxide (ENO) concentration is decreased in SCA patients with history of acute chest syndrome (ACS). A polymorphism in the NOS 1 gene was present in SCA children with diminished ENO and may underlie this finding. Healthy adults increase ENO in response to arginine intake, but it is not known whether children with SCA and ACS history respond similarly. We hypothesize that SCA children with ACS history are not able to increase ENO after oral arginine intake.
Methods: Three groups were enrolled from the Nemours Children’s Clinic hematology clinic: sickle cell patients with (ACS+), without history of ACS (ACS-), and healthy controls (HC). Exclusion criteria include asthma, smoking, allergy, congenital heart disease, or intercurrent illness. Exhaled NO levels were measured before and at 0.5 hour intervals after oral intake of 0.1, 0.2, or 0.4 g / kg of L-arginine. Vital signs, SpO2, spirometry, and plasma levels of arginine, citrulline, and ornithine were recorded before and 2 & 4 hours after oral intake L-arginine.
Results: To date, 14 patients have completed all doses yielding 42 patient days (4 HC, 6 ACS-, and 4 ACS+). Thus far there are no significant differences at baseline between groups with respect to age, ENO, arginine, citrulline, ornithine, spirometry, vital signs or oxygen saturation. After L-arginine administration at 0.1 g / kg, a trend toward enhanced ENO production was noted in the ACS+ group when compared with ACS- and HC groups (p=. 10). A subsequent, sharp decrease in ENO to below baseline followed in the ACS+ group not seen in other groups. Much smaller, equivalent percentage increases were noted in all groups at the higher doses tested. Significant increases in arginine, citrulline, and ornithine were noted in all groups, at all doses after arginine intake. The remaining physiologic parameters did not change significantly.
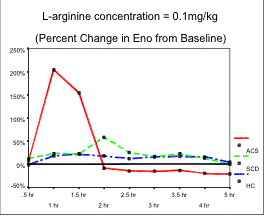 Conclusions:
Study enrollment continues but trends we
are following include our observation that SCA ACS+ patients may increase ENO
at least as well, if not better, than ACS- and HC patients. Additionally, there
may be dose dependency with lower doses producing more prominent ENO increase.
This would be in agreement with work by other investigators demonstrating
variability of plasma NO metabolites with different doses of oral L-arginine.
Lastly, steep decline in ENO may imply presence of accelerated airway NO
destruction in the airway.
Conclusions:
Study enrollment continues but trends we
are following include our observation that SCA ACS+ patients may increase ENO
at least as well, if not better, than ACS- and HC patients. Additionally, there
may be dose dependency with lower doses producing more prominent ENO increase.
This would be in agreement with work by other investigators demonstrating
variability of plasma NO metabolites with different doses of oral L-arginine.
Lastly, steep decline in ENO may imply presence of accelerated airway NO
destruction in the airway.
Intrapulmonary Percussive Ventilation (IPV) Improves Atelectasis in Intubated Pediatric Patients More Effectively Than Percussion with Postural Drainage (P&PD).
CL Hubble, KE Stevenson, and LH Lowe
Pediatric Critical Care Medicine, Respiratory
Therapy, and Radiology
Children’s Mercy Hospitals and Clinics
Kansas City, MO. 64108
Introduction: Atelectasis is a significant problem for ventilated pediatric patients that may prolong the need for mechanical ventilation and increase intensive care unit length of stay. This study compared intrapulmonary percussive ventilation (IPV) and percussion with postural drainage (P&PD) for the treatment of atelectasis in intubated pediatric patients.
Methods: Intubated patients with atelectasis were randomly assigned to receive IPV or P&PD treatments within four hours of atelectasis identification on chest radiograph. Four to seven treatments were given during the 24 hour study period. IPV treatments were delivered using the IPV-1/ Impulsator (Bird Products Corp., Palm Springs, CA.). Maximum pressure of 8 cmH2O greater than the baseline PIP, frequency cycles from 300 down to 100 per minute and 20 cc of normal saline were used during each 20 minute IPV treatment. The 20 minute P&PD regimen included three different clapping positions. Standardized ventilator settings were used during each treatment and non-treatment ventilation was determined by the clinical team. A radiologist blinded to the treatment modality scored atelectasis for pre-study and post-study chest radiographs using a four point scale. (0 = no atelectasis and 4 = multilobar atelectasis)
Results: IPV resulted in a greater improvement in atelectasis score than P&PD (two-sided Fishers Exact Test, p< 0.001). All patients receiving IPV (n=17) had a reduction in atelectasis score and 12 of 17 (71%) IPV patients had an improvement in score of ≥ 2. None of the patients receiving P&PD (n=16) demonstrated improvement and the atelectasis score worsened in four of 16 (25%) P&PD patients. Average pre-treatment atelectasis score was higher in the IPV group (2.9 vs 1.9, p = .001). There was no difference between groups in highest PEEP, number of treatments, age, diagnosis or sex. No complications were identified in either group.
|
Atelectasis Score Change |
P&PD |
IPV |
|
-2 |
1 |
- |
|
-1 |
3 |
- |
|
0 |
12 |
- |
|
1 |
- |
5 |
|
2 |
- |
8 |
|
3 |
- |
4 |
|
Totals |
16 patients |
17 patients |
Conclusions: P&PD was not effective in the treatment of atelectasis in this population of intubated, mechanically ventilated pediatric patients. Atelectasis improved in the patients treated with IPV suggesting that IPV may be an effective treatment of atelectasis in the ventilated pediatric patient. Further research investigating the treatment of atelectasis should compare IPV with standard ventilation strategies and other recruitment methods.
(Equipment support provided by Novametrix Medical System, Inc., Wallingford, CT)
Non-Invasive Carbon Dioxide Monitoring in Infants and Children with Congenital Heart Disease: End-Tidal versus Transcutaneous Techniques
JD Tobias MD
Departments of Anesthesiology & Pediatrics, University of Missouri, Columbia, Missouri
Introduction: The measurement of the partial pressure of carbon dioxide (PaCO2) is performed to evaluate the efficacy of ventilation. ABG analysis provides only a single measurement of what can be an ever-changing clinical picture. Commonly used non-invasive monitors of PaCO2 include end-tidal (ET) and transcutaneous (TC) devices. ETCO2 monitoring may be inaccurate with smaller tidal volumes, the site of sampling, the type of mechanical ventilation (intermittent versus continuous gas flow), ventilation-perfusion mismatch, and other cardiorespiratory issues, which may be present in congenital heart disease (CHD). The current study prospectively compares the accuracy of ET and TC CO2 monitoring in infants and children with CHD.
Methods: This study was approved by the hospital’s IRB and
verbal consent obtained. Patients
undergoing repair or palliation of CHD were included. ET-CO2 was monitored with a side
stream-aspirating device placed between the ET tube and the anesthesia
circuit. TC-CO2
was monitored using a TC-CO2/O2 device applied to the
anterior surface of the thigh or forearm.
Prior to placement, the TC electrode was cleaned, a new membrane
applied, and calibrated against a test gas. The working temperature was 45oC. When ABGs were obtained, the TC, ET,
and PaCO2 values were recorded. ABGs were measured at 37oC. The differences between the ABG and
both the TC and ET readings were compared using a non-paired t-test. A chi-square analysis and a two-way
contingency table was used to compare the number of ET and TC CO2
values whose absolute difference was < 2 mmHg and < 5 mmHg
from the PaCO2. To
avoid biasing the data by over-representing patients with multiple ABG
analyses, the data for each patient was averaged and counted as one data
point. All data are expressed as
the mean + SD.
Results: The cohort for the study included 53 patients (age: 4.0 + 5.9 yrs, weight: 19.1 + 23.9 kgs). The TC to PaCO2 difference was 2 + 1 mmHg. The ET to PaCO2 difference was 5 + 3 mmHg (p<0.0001). The TC to PaCO2 difference was < 2 mmHg in 30 of 53 patients and < 5 mmHg in 53 of 53 patients. The ET to PaCO2 difference was < 2 mmHg in 9 of 53 patients and < 5 mmHg in 30 of 53 patients (p<0.0001). The TC value was closer in 39 patients, the ET value closer in 6 patients, and both were of equal accuracy in 8 patients. No difference in the accuracy of TC monitoring was noted based on the patient’s age or type of CHD (cyanotic versus acyanotic). The difference between the ET and PaCO2 was greater in patients with cyanotic versus acyanotic CHD (7 + 3 mmHg versus 4 + 2 mmHg, p<0.0001) and in younger patients. The ET versus PaCO2 difference in patients 0-1 year of age, 1-5 years of age, and 6+ years of age was 6 + 3 mmHg, 4 + 3 mmHg, and 4 + 2 mmHg respectively.
Conclusions: In patients with CHD, TC-CO2 monitoring provided a more accurate estimate of PaCO2 than ET-CO2 monitoring. This difference was most pronounced in patients with cyanotic CHD and in patients less than 1 year of age. Since both non-invasive monitors offer distinct advantages, their maximal efficacy will likely be achieved when used to compliment rather than exclude one another. However, when close control of PaCO2 is necessary, TC monitoring should be considered.
Quantifying Diaphragm Function with Ultrasound:
Development in a Piglet Model of Diaphragm Fatigue
KC Kocis MD MS1, CA Kuroda BS1, WI Sternberger PhD2, JA Michael MS2, LC Ramac-Thomas PhD2, SM Daniels MS2, SR Aylward PhD3, J Kim PhD3, DG Nichols MD4,
J Gotsis MD5, PV Sackey MD5, LI Eriksson MD PhD5, PJ Radell MD PhD5.
1Dept of Pediatrics and 3Radiology
The University of North Carolina, Chapel Hill, NC; 2Johns Hopkins
University Applied Physics Laboratory Laurel, MD; 4Dept of
Anesthesiology and CCM, The Johns Hopkins Medical Institutions, Baltimore, MD; 5Dept
of Anesthesiology and Intensive Care, Karolinska Hospital and Institute
Stockholm, Sweden
Introduction: The objective of this study was to develop a noninvasive ultrasound metric to quantify diaphragm function in a piglet model of diaphragm fatigue.
Methods: 4 piglets(~20 kg) were studied using a well described model of diaphragm fatigue. Max transdiaphragmatic pressure(Pdi) and a novel ultrasound metric were obtained under baseline and fatigue conditions. Supramax transvenous phrenic nerve stimulation was used to induce fatigue. Digital ultrasound images were obtained in a standard right lateral sagittal plane. The Insight Toolkit for medical image segmentation and registration was used to register the end expiratory (exp) and end inspiratory (insp) hemidiaphragm (dia) position using a reference vertebral body. Quantification of a normalized area displacement was performed by 1)obtaining a leading edge curve fit of the exp and insp dia positions; 2)calculating the area between the exp and insp dia positions; and 3)normalizing this to the arc length squared of the exp dia.(figure 1).
Results: Pdi decreased from 41±18 to 26±12* cm H20 during fatiguing conditions while Normalized area di decreased from 0.0927±0.0084 to 0.0346±0.0106* (*p<0.05).
Conclusions: Normalized area di, a novel ultrasound metric, can be used to quantify diaphragm function in a piglet model of diaphragm fatigue.
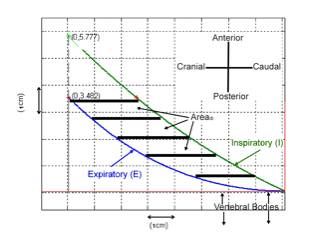
Figure 1 An illustration of the procedures for calculating normalized Area di. The area displacement (Area di) is measured between the insp (I) and exp (E) polynomial curve fits confined to the area within the exp curve (red stars) and then normalized to the arc length squared of the exp curve (E).
Variation in Practice for Severe Asthma
SL Bratton MD MPH, F Odetola MD, JL McCollegan, FH Levy MD MBA
Pediatric Critical Care, University of Utah, Salt Lake City UT, Pediatric Critical Care, University of Michigan, Ann Arbor, MI., Quality Consultant St Louis Children’s Hospital, St Louis, MO, Pediatric Critical Care, Washington University, St Louis, MO.
Introduction: Asthma is the most common chronic condition of childhood and a common reason for admission to a pediatric intensive care unit (PICU). However many providers differ in their care of critically ill asthmatic children.
Methods: We queried the Pediatric Health Information System database of the Child Health Corporation of America which contains member pediatric hospitals’ standardized data on diagnoses, procedures, interventions and outcomes. PICU admissions (primary ICD9 code of 493.0-493.9) for asthma during December 1999 through December 2003 from 29 hospitals with were included. The database includes extensive charge data allowing capture of therapies that generate charges. Data are presented as median with 25th and 75th quartiles and percentages.
Results: Of 7125 patients identified, 60% were male. The median age was 5.9 years (2.4-10.7 years). The median PICU length of stay was 1 day (1-2 days) while the length of hospital stay was 3 days (2-5 days). The median hospital charges were $11,900 ($7725-$19,615. Therapies in addition to oxygen, steroids and inhaled beta agonists included ipratropium bromide (n = 4193, 59%), terbutaline (n = 1841, 26%), heliox (n = 740, 10%), ketamine (n = 600, 8%), aminophylline (n = 436, 6%), and bipap ventilation (n=185, 3%).
1024 (14%) children received mechanical ventilation for a median duration of 1 day (1, 4 days). Their median hospital charges were $26,798 ($13,908-$58,958). Use of mechanical ventilation among centers varied from 0% to 61% of admissions with a median of 12% (6%-18%). Of the 185 children treated with bipap, 142 (77%) did not subsequently receive conventional mechanical ventilation and their median hospital charges were similar to patients treated with invasive ventilation [median $26,367, ($18,497-$37,006)]. Among patients who received either bipap or mechanical ventilation, additional therapies included ipratropium bromide (74%), ketamine (35%), terbutaline (23%), heliox (17%) and aminophylline (16%).
Conclusion: Treatment of severe asthma differs substantially between pediatric ICUs. Guidelines such as the recent NHLBI WHO-Sponsored Global Initiative on Asthma recommend administration of inhaled beta agonists, inhaled anticholinergic agents, systemic corticosteroids and oxygen and if the patient is not improving, then consider administration of aminophylline or subcutaneous, intramuscular, or intravenous beta agonist therapy. Consideration of mechanical ventilation is included as the final recommendation; however, criteria for intubation are somewhat vague (e.g. severe drowsiness, confusion, paCO2 > 45 mm Hg). Care of critically ill asthmatic patients may be improved with full adherence to guidelines and better delineation of appropriate indications for mechanical respiratory support.
Hyperglycemia in Critically Ill Children
EVS Faustino and M Apkon
Department of Pediatrics, Yale University School of Medicine, New Haven, CT
Introduction: Hyperglycemia which frequently occurs in critically ill adults contributes to increased mortality and morbidity in this patient population. Its prevalence in critically ill children, however, is not known. Moreover, the impact of hyperglycemia on patient outcome in the pediatric age group may differ from the adult population as a consequence of different metabolic demands, co-morbid conditions and age-dependent factors. This study aims to determine the prevalence of hyperglycemia among critically ill non-diabetic children and to correlate it with mortality and length of stay in the Pediatric Intensive Care Unit (PICU).
Methods: A retrospective cohort study was performed by reviewing computerized logs of point-of-care blood glucose measurements, hospital administrative databases and a computerized information system containing data for all patients admitted to our 11-bed multidisciplinary PICU from October 2000 to September 2003. Data were collected to describe blood glucose values, survival, and PICU length of stay for all patients for which at least one glucose measurement was obtained during their PICU stay. Patients were excluded if they were admitted to the PICU with diabetes mellitus. The prevalence of hyperglycemia was computed based on the first PICU glucose measurement and the highest within 24 hours and within 10 days of the initial value. Mortality rates and PICU lengths of stay among survivors without tracheostomy were compared using relative risks (RR) with 95% confidence intervals (CI) and Mann-Whitney test, respectively.
Results: A total of 942 patients were included
with median age of 3.2 years (inter-quartile range: 0.3-10.8 years). 36 of these patients died yielding a mortality rate of 3.8%. Using three cut-off values (120 mg/dL,
150 mg/dL and 200 mg/dL), the prevalence of hyperglycemia for the entire study
population ranged from 16.7% to 75.0%. The initial glucose did not correlate
with in-hospital mortality. In contrast, patients were more likely to die if
the highest glucose value within 24 hours of the first measurement was > 150
mg/dL (RR: 2.50; CI: 1.26-4.93).
The risk of dying was likewise increased 5.68-fold if the maximum
glucose within 10 days of the first was higher than 120 mg/dL (CI:
1.38-23.47). PICU length of stay
among survivors without tracheostomies was decreased for admission glucose
above threshold values of 120 mg/dL and 150 mg/dL. However, for all threshold values for maximum glucose within
10 days, hyperglycemics had significantly longer PICU stays. The other measures of hyperglycemia
were not significantly associated with differences in PICU lengths of stay.
Conclusions: Hyperglycemia occurs frequently among critically ill non-diabetic children and positively correlates with risk of in-hospital mortality and PICU length of stay.
Hyperglycemia in Pediatric Post-Operative Cardiac Patients
C Landers MD and W Douglas MD
University of Kentucky, Departments of Pediatrics and Surgery, Lexington, KY
Introduction: Hyperglycemia is known to be one of the predictors of pediatric ICU mortality. A recent adult study showed that tight control of serum glucose using an insulin infusion to maintain serum glucose between 80 and 110 mg/dl can decrease the incidence of patient mortality and morbidity (such as incidence of sepsis, need for renal replacement therapy or blood transfusion). The objective of this study was to examine the relationship between hyperglycemia and outcomes of pediatric post-operative cardiothoracic patients. In addition, we aimed to determine the number of pediatric cardiothoracic ICU patients that demonstrate hyperglycemia (>110mg/dl) and would therefore require insulin to achieve tight control of serum glucose. This was a pilot study to aid in sample size determinations for a possible prospective randomized trial to evaluate the effects of tight serum glucose control in pediatric ICU patients.
Methods: This was a retrospective chart review approved by the medical IRB of the University of Kentucky. All patients who underwent cardiothoracic surgery from August 2000 through July of 2003 were potential candidates for enrollment. Patients were included if they were less than 18 years old, were admitted to the PICU post operatively, and were intubated and ventilated upon arrival to the PICU. Patients were excluded if they were 18 years of age or older, were extubated prior to arrival to the PICU, had surgery in or returned to the NICU post operatively, or received insulin during their ICU stay. Information collected from the medical record included length of hospital stay, length of PICU stay, length of positive pressure ventilation (PPV), cardiopulmonary bypass time, risk of mortality (using the RASCHE score), all serum glucose measurements done while in the PICU, the patient’s need for renal replacement therapy and packed RBC transfusions, incidence of infection and survival to hospital discharge.
Results: A total of 247 charts of cardiothoracic patients less than 18 years of age were reviewed. Eight-five of these were excluded from the study (42 were not ventilated upon arrival to the PICU, 37 were operated on in the NICU, 3 received insulin during their ICU stay, 3 died in the OR). Of the resulting 163 patients, 156 (97.5%) had at least one serum glucose greater than 110mg/dl and none had a previous diagnosis of diabetes mellitus. The median number of days patients had at least one measured serum glucose above 110mg/dl was 3 (range 0-28). There were only four patients whose glucose level never exceeded 110mg/dl. The patients experienced hyperglycemia on 61% of all PICU days (median, range 1-100%). Nine (5.5%) of the 163 died during the hospitalization, 4 (2.5%) received RRT, 51 (31.3%) received packed RBC transfusions, 26 (15.9%) had a bacterial infection. The median PICU length of stay for survivors was 5 days with a range of 2-100 and an average of 8 days ± SD12. The median length of PPV for survivors was 2 days with a range of 1-58 and an average of 4 days ± SD7. After controlling for risk of mortality and bypass time, serum glucose levels above 235 were associated with mortality and above 150 with the need for RRT. Serum glucose levels were not associated with length of PICU stay, length of PPV, need for transfusion or occurrence of infection.
Conclusions: A majority of pediatric post operative cardiac patients would qualify for insulin administration in order to maintain serum glucose between 80-110 mg/ml. Elevated glucoses are associated with need for RRT and risk of death. A prospective randomized trial is reasonable to better understand if controlling hyperglycemia in the post operative pediatric cardiac patient will improve these outcomes.
Reducing the Specimen Redraw Rate in a Pediatric Intensive Care Unit
JL McCollegan, A Cleary RN, B Markovitz MD
Office of Pediatric Quality Management, Pediatric
Intensive Care Services,
Departments of Pediatrics and Anesthesiology
St. Louis Children’s Hospital and Washington University School of Medicine
St. Louis, MO
Introduction: We used a newly developed PICU medical error reporting tool (1) to identify and prioritize laboratory error reports. A significant proportion of these errors involved the need for a specimen redraw, which we consider to be a patient safety issue (as additional blood loss and the potential for a needle stick occurs). Through drill-down analysis a multi-disciplinary group of stakeholders identified two main areas of opportunity focused on specimen collection: proper equipment and appropriate technique. We describe here a pre/post intervention to reduce the rate of specimen redraws in our PICU.
Methods: This project was conducted in a multi-disciplinary, 26 bed pediatric intensive care unit with approximately 44,000 lab specimen draws per year. An independent data collection was undertaken to determine the specimen redraw rate, defined as the number of redraws required (e.g., specimen clotted, hemolyzed, mislabeled, etc) as a proportion of total specimens drawn within the last 24 hours. Six weeks prior to implementation of any interventions, the team conducted audits recording the specimen redraw rate and the reason for each redraw. Our primary intervention was education of the PICU nursing staff conducted by the laboratory technicians and supervisors. This was a 2 stage intervention; the first stage focused on reinforcing proper equipment (e.g., collection tubes and needles) and appropriate specimen collection technique while the second stage focused on appropriate utilization of phlebotomy staff (e.g., for capillary sampling) and a repeat of appropriate specimen collections. During both stages, supplemental educational tools were provided such as posters and bedside flyers focused on helpful tips for avoiding common technical mistakes.
Results: Prior to implementation, the audits revealed a specimen redraw rate of 2.2%. After the first stage intervention, the specimen redraw rate was 1.2%. Following the second stage of intervention the specimen redraw rate declined to 0.7%. This performance has been consistent for over eight months. Data collection continues to monitor for sustained improvement and to quickly recognize any drop in performance.
Conclusion: Using our new error reporting tool, we have identified potentially unnecessary patient blood sampling as a previously unappreciated aspect of patient safety. In decreasing the measured redraw rate from 2.2% to 0.7%, we have eliminated the need for approximately 660 laboratory specimen draws from our most critically ill patients.
Reference:
1. Levy, FH, McCollegan, J., Scholl, P., Cohen, A. A New Error Reporting Tool in a Pediatric Intensive Care Unit (PICU). Pediatric Critical Care Medicine. 2003; 4: A93.
A Comparison of Diabetic Ketoacidosis Orders Generated by a Computerized Program versus Traditional, Handwritten Method
VU Vaidya MD, KG Crawford-Bell MD
Division of Pediatric Critical Care, University of Maryland School of Medicine, Baltimore, MD
Introduction:
Diabetic ketoacidosis (DKA) in
children is a life-threatening condition that requires timely and specific
management. Treatment involves precise intravenous fluid calculations, accurate
dosing of insulin, and frequent changes in therapy making the management
process prone to errors. Incorrect calculations of insulin dosage or
intravenous fluids containing high concentration of potassium could result in
serious adverse events or even death. The goal of this project was to develop a
computerized program for emergency management of DKA, and to compare it to the
traditional handwritten orders in a simulated test environment using written
case scenarios. The objectives were to determine if the computerized program
would be faster and more accurate than hand-written orders.
Methods: Pediatric housestaff who volunteered for the study were randomized to either the handwritten group or the computerized group. Each subject was then given two different DKA case scenarios and was asked to generate fluid and insulin orders using either the handwritten or computerized method. The handwritten group had access to a calculator, to a pre-printed protocol for DKA management currently in use at our institution, and a body surface area nomogram. The computerized group had access only to the computerized program which required the user to input patient demographics, laboratory values, and clinical estimate of dehydration. Based on this input, the computerized program generated orders according to the institutional protocol for DKA management. Each subject’s time-to-order-submission and correctness of orders (based on predetermined criteria) was recorded. In addition, subjects who used the computerized method were asked to evaluate the usefulness of the computer program. Statistical comparisons were made using a two-tailed Student’s t-test.
Results: Twenty-nine subjects volunteered for the study. Via block randomization within the year of residency training, 18 (62%) subjects were randomized to the computerized group and 11 (38%) were randomized to the handwritten group. The computerized group completed their orders in an average of one minute and 21 seconds while the handwritten group required an average of ten minutes and 35 seconds (p<0.0001). Accuracy was measured by evaluating the percentage of correct orders for each group. One hundred percent of computerized orders were determined to be correct, while 65.5% of the handwritten orders were correct (p<0.0001). Of the 18 subjects in the computerized group, 15 had previous experience using the institutional pre-printed protocol for DKA management during their training. They were asked to compare the two methods and indicate their method of choice. All 15 subjects (100%) stated their preference for the computerized method over the handwritten method.
Conclusions: Orders generated using a computerized program were significantly more accurate and were obtained far more quickly than those generated by the traditional, hand-calculated and handwritten method. User acceptance of the new computerized method was excellent as all users indicated unanimous preference for the computerized program. The use of such programs could improve patient safety and enhance efficiency of management. An educational component built into the program ensured that the providers did not lose the opportunity to understand the principles and basis of management that were used in generating the computerized orders.
Do Children Share Critical Care Resources with Adults? A Descriptive Analysis
FO Odetola, SJ Clark, SL Bratton, MM Davis.
Department of Pediatrics and Communicable Diseases, University of Michigan Health System, Ann Arbor, MI.
Introduction: Prior studies have reported improved morbidity and decreased resource use for adolescent trauma victims admitted to a pediatric intensive care unit (PICU) versus an adult surgical intensive care unit. However, no prior studies have characterized the prevalence and characteristics of critical care facilities in which children share resources with critically ill adults.
This study was conducted to describe co-residence of critically ill adult and pediatric patients in US PICUs.
Methods: In January through May 2004, we conducted a cross-sectional survey of medical directors of all known US critical care facilities for children other than preterm neonates.
Results: Fourteen (5.5%) of the 257 responding PICUs reported co-residence of critically ill pediatric with critically ill adult patients. These “co-resident” PICUs had a median number of 4.5 beds (interquartile range [IQR]: 2-10). Eleven (79%) of the units had at least one pediatric intensivist covering the unit, with 24-hour coverage by a pediatric intensivist occurring in 8 of the units. All the co-resident PICUs had the capacity to provide mechanical ventilation, vascular pressure and intracranial pressure monitoring for their pediatric patients, while hemodialysis, hemofiltration, and nitric oxide therapy could be performed in fewer settings (71%, 57%, and 79% of the PICUs, respectively).
Conclusion: Co-resident PICUs are often staffed by pediatric intensivists and provide core critical care services, but lack other advanced therapeutic modalities that may influence patient outcomes. Although there are currently few co-resident PICUs nationally, the phenomenon of co-residence warrants further study because constrained resources may force some smaller facilities that currently have separate PICU beds to consider co-residence in the future.
Availability of Advanced Therapeutic Modalities in US PICUs – 2004
FO Odetola, SL Bratton, SJ Clark, MM Davis.
Department of Pediatrics and Communicable Diseases, University of Michigan Health System, Ann Arbor, MI.
Introduction: The care of critically ill children often requires the use of advanced therapeutic modalities. For adults, the availability and amount of technological support in an intensive care unit have been associated with reduction in hospital mortality. Better understanding of the distribution of technology within US pediatric intensive care units (PICUs) might provide opportunity to impact outcomes of PICU care. This study was conducted to describe the distribution of 7 advanced therapeutic modalities among US PICUs.
Methods: In January through May 2004, we conducted a cross-sectional survey of medical directors of intensive care facilities for children other than preterm neonates.
Therapeutic modalities studied included mechanical ventilation, invasive vascular and intracranial pressure (ICP) monitoring, renal replacement therapy (hemodialysis and hemofiltration), and inhaled nitric oxide therapy.
Results: We received responses from 257 of 337 eligible PICUs (response rate = 76%). All of the responding PICUs reported the ability to perform mechanical ventilation and invasive venous and arterial pressure monitoring. 247 PICUs (94%) had ICP monitoring available, while 199 (77%) could provide inhaled nitric oxide therapy. Hemodialysis was available in 195 (76%), and hemofiltration in 187 (73%) of the PICUs. Availability of advanced therapeutic modalities generally increased with greater numbers of PICU beds; for example, 96% of PICUs with 20 or more beds had hemodialysis available compared to 43% of PICUs with 6 or fewer beds (p<0.01).
Conclusion: The availability of advanced therapeutic modalities in PICUs nationally varies by technology and by PICU size, defined by number of beds. The need for advanced therapeutic support for critically ill children may prompt inter-PICU referrals. The impact of the availability of advanced care modalities on referral patterns and clinical outcomes for critically ill children warrants further study.
Use of a Web-based Tool to Enhance Medical Student
Learning
in the Pediatric Intensive Care Unit and Inpatient Wards
FA Maffei MD,1 EB Nazarian MD,2 P Ramnarayan MD,3
NJ Thomas MD,4 JS Rubenstein MD 2
Divisions of Pediatric Critical Care: 1 Janet Weis Children’s Hospital, Danville, PA; 2 Golisano Children’s Hospital at Strong, Rochester, PA; 3 Great Ormond Street Hospital for Children, London, UK; 4 Penn State Children's Hospital, Hershey PA.
Introduction
Numerous web-based clinical tools exist and are easily accessible to medical students. The educational value of these tools remains largely untested. ISABEL is a web-based pediatric differential diagnostic tool developed by the ISABEL medical charity in the United Kingdom. Using sophisticated textual pattern recognition software to search through standard pediatric texts, ISABEL generates a differential diagnosis after patient data has been entered. A recent study demonstrated that ISABEL displayed at least one ‘additional clinically important’ diagnostic possibility in 85/206 cases admitted to pediatric intensive care units (41.3%, CI 34.6-48%). We hypothesized that ISABEL may serve as a useful adjunct to improve the ability of medical students to generate an appropriate differential diagnosis.
Methods
After IRB approval was obtained, consented medical students were randomly assigned to two groups: ISABEL usage group or a control group. ISABEL students were allowed access to the web-based tool in addition to traditional resources to generate differential diagnoses. They were given confidential passwords, an introduction to the website and were strongly encouraged to use the tool with each new patient encounter. The control group did not have access to the site and used traditional sources to generate differential diagnoses. Objective analysis of the tool was done using a standardized post-rotation test given to all students. A post-study questionnaire and interviews provided qualitative data.
Results
Forty-three students (22 ISABEL and 21 control) were enrolled. Of the 22 students in the ISABEL group, 15 completed the trial. Student analysis of the tool was positive overall; 12/15 students (80%) reported the tool was as helpful or more helpful when compared to traditional resources, 10/15 students (66%) reported ISABEL often or always provided an additional diagnosis not initially considered. There was no difference in post-rotation test scores between the two groups (ISABEL 73 vs. control 74, p = .67). All students interviewed agreed that the use of web-based tools should be incorporated into medical education after formal evaluation and validation of such tools.
Conclusion
Medical students using ISABEL were able to expand their differential diagnoses the majority of times they used the tool. They found the tool easier to use than traditional texts. The lack of an objective difference between groups was likely due to the small numbers and lack of a sensitive test to detect true differences. Web-based tools can be an important instrument in medical education but require formal evaluation and validation prior to their use.
The FIVE Critical Issues that Define Your Critical
Care Unit:
How to “SCORE in Healthcare™”
J Meliones*, J Levy, C Shay, S Sloate. PracticingSmarter, DurhamNC, *UNCChapel Hill
Based on our experience implementing performance management solutions in over 30 institutions and analyzing tens of thousands of surveys, several consistent principles of performance improvement have been identified. For the organization to be successful, they must appropriately monitor and manage the key initiatives that align and drive behavior consistent with five basic principles. Using our targeted approach, we have demonstrated an increase in approval rating in administration from 46% to > 90%. This occurred while increasing the contribution margin by >$5M. We present a systematic approach for these five critical issues which allows you to conquer them and “SCORE in healthcare™”.
1.Safety: Quality & safety have become a major focus in the intensive care unit. From our data base, we found that staff productivity correlated strongly with medication error rate (r=.86) while quality correlated with a “Patient Engagement Index” (r=.8). An inherent imbalance may occur between obtaining safe, high quality care & achieving a healthy bottom line. To pursue a healthier balance, systematic improvements must be implemented. These include staff engagement initiatives, a systematic safety reporting/prevention solution & CPOE.
2.Communication: Staff satisfaction correlated positively with understanding and supporting the goals of the administration (r=.86). However, this did not correlate with turnover rate, which only correlated with staff perception of workload and pay rate (r=.76). These data highlight the importance of a direct and honest dialogue when leaders are monitoring and communicating workload / pay rate issues to their staff.
3.Operations improvement: Operations improvement must focus on the key drivers of financial success in the ICU. A primary indicator of ICU financial performance is the contribution margin (CM). CM identifies the ability of the ICU to cover its variable costs and contribute to the fixed overhead of the institution. CM and days cash on hand have strong positive correlations with referring/staff physician satisfaction (>.9). Managing the processes that underlie these indicators is essential for financial success of the organization and achieving high referring physician satisfaction. The primary means of improving CM are through increasing net revenue (↑ number of patients, ↑ contract pricing, & improved collections) and most importantly, managing variable costs (managing staffing & supplies to volume). One service line was able to increase their contribution margin by >$15M (> 200% increase) over 5 yrs using this approach. Another institution achieved an ROI of 20 times by focusing on accounts receivable, rebilling denials and more aggressively pursuing collection accounts.
4.Rewards: Rewards / pay for performance remain the single most significant motivator for performance. Over 90% of faculty and staff would support a significant cost or management initiative if they would directly benefit from the initiative in the form of; a direct cash infusion, a reduction in their workload or time, or a demonstrable improvement in care.
5.Entrepreneurialism: Rewarding entrepreneurialism remains a powerful yet underutilized motivator for innovation. For technology transfer to be effective, many impediments must be overcome including; who owns & how to protect the IP, what is copyrightable vs. patentable, the reward structure of the individual vs. institution, how do you fund the initiative and many other factors that may alienate clinicians and cause them to become disenfranchised.
To SCORE, leaders must keep these concepts forefront in their daily decisions while attempting to balance the seemingly disparate motivations of key stakeholders. After all, even the most extraordinary effort is meaningless if directed at the wrong targets for change.
A Computerized Program for Changing from Rule of Six to Standardized Drips
VU Vaidya MD, MI Gaffoor MD, E Hilmas PharmD, and L Mathews RN
Division of Pediatric Critical Care, University of Maryland School of Medicine, Baltimore, MD
Introduction: Continuous infusions used in the PICU and NICU are most often compounded using the ‘Rule of Six’ (RO6) method, which results in a unique concentration for each patient weight. A recent mandate by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) requires hospitals to abandon the RO6 method in favor of the ‘Standard Concentration’ (SC) method, limiting the choice to only a few standard concentrations. Barring organizations that have been using the SC method prior to the mandate and those that have recently changed, for the remainder, compliance with the mandate is proving to be a daunting task. This is not surprising, given the wide range of weights and the need for precise control of fluid balance in pediatric and neonatal patients. The complexity is further increased as the shift to SC method necessitates a change at multiple levels involving physicians, pharmacists, and nurses. Our project goals were to develop a simplified, yet comprehensive computerized solution that would facilitate easy transition to the SC method, making it applicable in other institutions as well.
Methods: The
solution was implemented in two steps. Step (A): Identification of ideal
standard concentrations. This was accomplished by developing a computerized
program, the “Concentration Optimizer” which automated the task of rapidly
generating two to four concentrations for each drug. An algorithm built into
the program generated the concentrations when the following parameters were
entered into the program; (i) minimum and maximum dose, (ii) dose titration
interval, (iii) lowest patient weight (iv) lowest pump infusion rate (ml/hour),
and (v) maximum acceptable fluid load resulting from the infusion.
Step (B): Development of a Computerized Physician Order Entry (CPOE) program for ordering continuous infusions. Using just two of the data entry fields (dose and patient weight) for any selected drug, the CPOE program automatically selected a single “best” concentration from the 2 to 4 available concentrations that were generated by the “Concentration Optimizer” in Step (A). The selected concentration was such that it resulted in acceptable fluid load based on fluid limits set by the user. Users could override the computer to select an alternate concentration. The program then generated a comprehensive printout order that contained compounding instructions for pharmacy, administering instructions for nurses and a weight-specific dosing chart displaying infusion rates at all dose ranges. Additional features such as dose range checks, drug information, and other safety alerts were built into the program.
Results: Using the Concentration Optimizer a list of two to four ideal concentrations was rapidly established for each of the forty drugs (www.icudrips.org/conc_list.html). The CPOE program enabled the user to order infusions by the SC method, requiring in fact fewer steps and no calculations, as compared to the RO6 method. The optimal concentration selected by the CPOE program resulted in clinically acceptable fluid load when tested for a wide range of weights (0.5 kg to 70 kg) and doses, for each of the forty drugs. The comprehensive printout instructions enabled the pharmacists and nurses to seamlessly transition to the new method.
Conclusions: The “Concentration Optimizer” and the “CPOE program” provided a
comprehensive yet simplified solution to implementing the SC method. We foresee
that the program could be easily exported and rapidly implemented in other
institutions. Standardizing the process across institutions would provide an
additional layer of safety in use of continuous infusions in pediatric and
neonatal patients.
Factors That Drive The Distribution of Pediatric Critical Care Services
FO Odetola, SJ Clark, MM Davis
Department of Pediatrics and Communicable Diseases,
University of Michigan Health System, Ann Arbor, MI.
Introduction: Pediatric intensive care units (PICUs) have grown in number and size over time in aggregate across the U.S. While some units have faltered and closed, others have emerged and expanded. The factors driving such changes have not been well characterized. This study was conducted to explore the factors that promote the development, expansion, or closing of a PICU.
Methods: A cross-sectional telephone interview of the knowledgeable officials from hospitals where PICUs were established, expanded, or closed between 1997 and 2001, as identified by comparison of the 1997 and 2001 AHA survey of hospital facilities.
Results: To date, 8 of 22 eligible institutions have completed telephone interviews, representing 5 PICU closures, 1 newly established PICU, and 2 PICU expansions. Based on the officials’ comments from these initial interviews, the establishment of PICUs is driven primarily by the need to care for patients requiring intensive cardio-respiratory monitoring and/or post-operative surgical care, and to respond to the needs of outlying community hospitals. The main factors that drive the expansion of PICU resources are growth in pediatric sub-specialist medical and surgical support for critically ill patients, and the need to match increases in demand and patient volume experienced by the PICU. With regard to PICU closures, external competition for both patients and subspecialty care providers within the same market, as well as lower than expected volume of patients and/or no positive financial margin, were cited as key factors.
Conclusions: The establishment, expansion, or closure of PICUs is driven largely by patient demand for pediatric critical care services. The availability of subspecialists, as well as competition between PICUs within the same market, influence the long-term sustainability of services for critically ill children. This study provides new insight into decision-making that influences the distribution of critical care services for children.
Parent Bed Spaces in the PICU: Effect on Parental Stress and Staff Perceptions
GC Hefley1, MNSc, RN; AB Smith2, PhD, RN, CPNP; KJS Anand3, MBBS, DPhil
1Clinical Nurse Specialist, PICU, Arkansas Children’s Hospital, Little Rock, AR; 2Director, Grants and Research Administration, Cook Children’s Medical Center, Fort Worth, TX; 3Professor of Pediatrics, Anesthesiology, Pharmacology & Neurobiology, Oakley Endowed Chair of Critical Care Medicine, University of Arkansas for Medical Sciences, Little Rock, AR.
Introduction: Family-centered care with parent bed spaces allowing continual parent presence has demonstrated many benefits in general pediatric care areas, but it is unknown if these benefits will occur in an intensive care environment. The construction of new PICUs with parent bed spaces is a recent trend, although no reports have investigated the effect of this new environment on the stress perceived by staff members or parents.
Objective: The purpose of this study was to examine the impact of new PICU environments with parent bed spaces on perceived stress by staff members. Research questions were: 1) What potential benefits / concerns do staff perceive about having parent bed spaces that allow continual parental presence in the PICU? 2) How do potential benefits and concerns identified by staff prior to completion of the new PICU compare with those experienced when caring for children and parents in the new PICU?
Methods: The pre- and post-survey comparative study design was used at Arkansas Children’s Hospital (ACH) and Cook Children’s Medical Center (CCMC), Fort Worth. Both hospitals expanded their PICUs with the inclusion of parent bed spaces. A non-random sample of registered nurses and respiratory therapists who provided direct bedside care in both old and new PICUs completed the Staff Benefit/Concern Scale (SBCS) and answered open-ended questions 1 month before and 3 months after moving to the new PICU. The SBCS scores 32 items via 5-point Likert scales with a high overall Inter-rater Reliability Coefficient (r=0.83) and four subscales: Parent Benefit (r=0.86), Staff Benefit (r=0.77), Parent Concern (r=0.74), Staff Concern (r=0.88).
Results: Staff members at both sites (ACH=36, CCMC=48) identified similar parent and staff benefits and concerns in the pre-move period with no significant differences in subscale means. Results from the post-move period demonstrated significant changes within group means at each site. ACH staff perceived greater benefits for staff (pre 3.05 vs post 3.47, p=0.006), decreased concerns for parents (pre 3.85 vs post 3.29, p=0.001) and staff (pre 3.39 vs post 2.65, p=.000). CCMC staff perceived decreased benefits for parents (pre 3.63 vs post 3.26, p=.028) and staff (pre 3.34 vs post 2.99, p=0.035), but no change in concerns for parents (p=0.257) or staff (p=0.88). Significant differences in the post-move subscale means between sites suggested greater benefits for parents (4.01 vs 3.26, p=0.000) and staff (3.47 vs 2.99, p=0.011), and decreased staff concerns (2.65 vs 3.49, p=0.000) at the ACH site compared to the CCMC site. These site differences may be related to an increase in the private rooms at ACH (pre 26%, post 85%) but a slight decrease in the private rooms at CCMC (pre 50%, post 36%). Major benefits perceived by ACH staff included greater assistance with patient care and emotional support provided by parents, whereas CCMC staff noted greater concern about patient confidentiality.
Conclusions: The inclusion of parent bed spaces in the PICU may increase the benefits perceived by staff members and significantly reduce their concerns, particularly in PICUs designed with predominantly private rooms. Including parent bed spaces in PICUs with open-bay areas may increase the stress perceived by staff members because of decreased parent and staff benefits and greater concern for patient confidentiality.
Comparison of Surveyed Expert Opinion on Unanswered Research Questions and Minimally Clinically Important Differences in Pediatric Cardiac Arrest Survival Outcomes
A Donoghue1, V Nadkarni1, L Nesbitt2, S Campbell2, M Osmond, RA Berg, and I Stiell2 for the CanAm Pediatric Cardiac Arrest Investigators
1Division of Critical Care Medicine,
Children’s Hospital of Philadelphia, PA
2Department of Emergency Medicine and Ottawa Health Research
Institute,
University of Ottawa, Ontario
Introduction: Pediatric out-of-hospital Cardiac Arrest (CA) is uncommon and has poor outcome. Clinical trials to evaluate promising CA interventions are needed. We surveyed an international group of experts as to important unanswered research questions and goals for improvement in outcomes yielded by an intervention trial. We hypothesized that physician specialty would influence surveyed opinion of the most appropriate targeted minimally clinically important differences (MCID) in survival outcomes following pediatric CA.
Methods: Using a
modified Dillman’s Total Design Method, our international, NIH-funded,
resuscitation consortium conducted two prospective mail surveys. 241 CA experts
from professional organizations in 15 countries were surveyed. In survey 1,
experts rated research question priority on a 5-point Likert scale, for each of
47 potential study questions presented in 4 domains (Emergency Medical Systems
[EMS], Ventilation, Circulation, and Post Resuscitation care). In survey 2,
these experts selected MCID for the effectiveness of an intervention on 4
standard Utstein clinical CA outcomes: Return of Spontaneous Circulation
(ROSC), survival to hospital admission, survival for 24 hours, and survival to
hospital discharge. Comparisons between groups were made by Wilcoxon rank sum
for ordinal data and chi square analysis for dichotomous data.
Results: Survey response rate was 56% for survey 1 and 52% for survey 2. Most (85%) respondents were physicians. For survey 2, most respondents practiced emergency medicine (EM) (43%) or pediatrics (34%). The majority of pediatricians were EM (28/36) or critical care medicine (CCM, 7/36) subspecialists. On survey 1, the clinical questions most highly prioritized were: Does the use of epinephrine improve outcome? (71%); What is the incremental impact of bystander CPR, rapid defibrillation and ALS interventions? (71%); What is the effectiveness of bag-valve mask vs. tracheal intubation vs. Laryngeal Mask Airway for ventilation during CA (71%); Does post resuscitation therapeutic hypothermia improve outcome? (68%); Does chest compression before defibrillation improve outcome? (65%). On survey 2, pediatric physicians were more likely than non-pediatric physicians to require higher MCIDs (8-10% vs. 2-6%) for improved 24 hour survival (p=0.006). EM physicians were more likely than non-EM physicians to require higher MCIDs (8-10% vs. 2-6%) for improved survival to hospital admission (p=0.039). In addition, EM physicians were more likely to select the outcomes of ROSC and survival to hospital admission as not clinically important (p=0.025). CCM physicians were more willing to accept lower MCIDs (2-6% vs 8-10%) for improved survival to hospital admission (p=0.039).
Conclusions: This is the first international survey of resuscitation expert opinion to define consensus interventions and specific MCID goals for clinical outcomes in pediatric CA. Differences in opinion regarding MCIDs for short term survival are related to training background. International expert surveys can assist planning consortia in multi-center trial design.
Assessment of the Extent of Code Blue Review in Pediatric Hospitals
R Berens, K Colpaert, S Percy, Jr, M Quasney, R Brilli, B McGarr, TB Rice and NACHRI PICU FOCUS Group.
Anesthesiology and Critical Care, Medical College of Wisconsin, Children’s
Hospital of Wisconsin; National Outcomes Center, Inc., Children’s Hospital and
Health Systems, Milwaukee, WI; Division of Pediatric Critical Care, Hackensack
University Medical Center, Hackensack, N.J.; Le Bonheur Children’s Medical
Center, Memphis, TN; Cincinnati Children’s Hospital and Medical Center,
Cincinnati, OH; St. Joseph’s Children’s Hospital of Tampa, Tampa, FL; Critical
Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin,
Wauwatosa, WI
Introduction: Pediatric cardiopulmonary arrests (code blue) are relatively rare events. Regulatory agencies require that hospitals review and assess their response to cardiopulmonary arrest events. Resources and personnel are designated to provide clinical care during an arrest and then to subsequently collect data and analyze the system’s effectiveness. Some hospitals utilize CPR QI review forms to capture the information needed to assess the performance of their code teams. The AHA has established essential data elements that they believe should be reviewed for these events. The purpose of this survey was to determine if the code blue QI review process differs between hospitals and to evaluate the extent of review that takes place; i.e. those asking many questions (rigorous) versus those asking relatively few questions (casual).
Methods: In 1996, the AHA established a list of practice guidelines that have been defined as both “essential” and “gold standard” as it relates to adult cardiac arrest. A NACHRI PICU FOCUS Group Codes subcommittee requested code blue QI review forms from NACHRI member hospitals. The QI review forms were categorized as either rigorous (asking many questions) versus casual (asking fewer questions). Hospitals were grouped as either having rigorous or casual review of their CPR process, based upon the number of questions asked. The number of questions determining the extent of review was the median number of questions asked by all the hospitals combined. QI forms were analyzed by two investigators (RJB and KDC). The questions were placed into categories of interest and analyzed between the rigorous and casual review processes. The questions asked by > 50% of the hospitals were analyzed separately between the two groups as well. A t-test was performed with significance defined by a p-value <0.05.
Results: 114 NACHRI member hospitals were surveyed. 84/114 (74%) surveys were returned. 30/84 (26%) of those hospitals returned QI forms. 15 hospitals utilized a casual review process and 15 utilized a rigorous review process. In aggregate, the QI forms from all 30 hospitals asked 283 questions in 20 different categories of interest. There were significant differences in 9 of the categories. Analysis of frequently asked questions showed that 28/283 (10%) of questions were asked > 50% of the time. 12/28 (42%) of the questions were asked significantly more in the rigorous review group. There was significantly more focus on the AHA essential data points in the rigorous review group (13% vs. 41% p < .0001) yet neither group assessed > 50 % of the essential elements.
Conclusions: There is significant variability in type of data requested on CPR QI review forms. This data demonstrates a lack of agreement between institutions regarding which types of questions should be used to analyze CPR processes and outcomes, despite AHA published guidelines for CPR review processes.
Frequency of Tiered Response Teams in NACHRI Institutions
S Percy Jr, K Colpaert, M Quasney, R Brilli, L Easterling, TB Rice, R Berens and the National Association of Children’s Hospitals and Related Institutions (NACHRI) PICU FOCUS Group
Division of Pediatric Critical Care, Hackensack University Medical Center, Hackensack, NJ; National Outcomes Center, Inc. Children’s Hospital and Health Systems, Milwaukee, WI; Division of Pediatric Critical Care, Le Bonheur Children’s Medical Center, Memphis, TN; Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH; Cook Children’s Medical Center, Fort Worth, TX; Pediatric Critical Care Division, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI; Anesthesiology and Critical Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI
Introduction: While cardiopulmonary arrests are rare events in pediatrics, there are many more instances when children require a higher level of assessment but not necessarily intensive care level services. Many institutions seem to be moving to tiered responses such as “Code Light”, “Med Alert”, or “reassistance”, in an effort to appropriately utilize scarce resources and potentially reduce the number of true “code blues”. Anecdotal reports from members of the NACHRI PICU Focus Group suggest that the number of true code blue situations decreases with the presence of tiered response teams.
Methods: A NACHRI PICU FOCUS Group subcommittee requested information from NACHRI member hospitals about the presence of a “tiered” response team in their institution. Responding hospitals were categorized as either a freestanding pediatric hospital (FSH) or a pediatric hospital-within-a-hospital (HIH) and then compared using Fisher’s Exact Test based on whether the facility had a tiered response in place.
Results: 114 NACHRI hospitals were surveyed regarding the presence of a “tiered response” process to address “noncode”situations. 53/84 (54%) hospitals answered questions about the presence of a tiered response team. 36/53 (68%) indicated that they had a tiered response. Responding institutions were nearly evenly divided between FSH (26) and HIH (27). There was no statistically significant difference between FSH and HIH with regards to the presence tiered response teams (p=0.2409). Multiple institutions indicated informal mechanisms for addressing pre-arrest situations including consultation with the ICU attending or critical care nurse.
Conclusions: While tiered response teams may be increasing in popularity with over two thirds of the institutions having some form of a tiered response, there is currently no difference between freestanding children’s hospitals and children’s hospitals-within-a-hospital in the frequency of these teams. Further investigation is needed to see if the perception that a tiered response team reduces the frequency of cardiopulmonary arrests is valid and if in those facilities where a tiered response is present there has been a reduction in the actual number of cardiopulmonary arrests.
Evaluation of Pediatric Code Cart Medications
K Colpaert, S Percy Jr, M Quasney, R Brilli, B McGarr, L Easterling, TB Rice, R Berens and NACHRI PICU FOCUS Group
National Outcomes Center, Inc. Children’s Hospital and Health Systems, Milwaukee, WI; Division of Pediatric Critical Care, Hackensack University Medical Center, Hackensack, NJ; Le Bonheur Children’s Medical Center, Memphis, TN; Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH; St. Joseph’s Children’s Hospital of Tampa, Tampa, FL; Cook Children’s Medical Center, Fort Worth, TX; Division of Pediatric Critical Care, LeBonheur Children’s Medical Center, Memphis, TN; Anesthesiology and Critical Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI
Introduction: As pediatric cardiopulmonary arrests are relatively rare events, it is important to stay current with national guidelines and practice suggestions. The American Heart Association (AHA) has published recommendations for medications to be used in Pediatric Advanced Life Support (PALS). Our purpose is to evaluate the first line medications recommended by the AHA as used by free-standing children’s hospital (FSH) vs. pediatric hospital with an adult hospital (HIH)
Methods: A NACHRI
PICU FOCUS Group subcommittee surveyed NACHRI member hospitals regarding
medications that are maintained on pediatric code carts. The presence of first
line AHA medications on code carts was recorded by the FOCUS Group. Hospitals
were then divided into either FSH or HIH to analyze differences between
medication lists. Students t-test was performed with
significance defined by a p-value <0.05
Results: 114 NACHRI hospitals were surveyed. 84/114 (76%) of distributed surveys were returned. 31/84(37%) of those hospitals responding to the survey included medication lists. Overall, 77 different medications were present on code carts. There are 12 medications currently recommended by the AHA/PALS as first line medications. The average number of medications present on the code carts was 23 (range 13 – 33). Atropine, epinephrine (both concentrations), lidocaine, narcan, and sodium bicarbonate were universally present. Other medications present on at least 90% of carts included adenosine, calcium chloride, dopamine, and glucose. Medications introduced as 1st line and alternative medications in the 2000 guidelines were present less often. These included vasopressin (32%, present but not recommended for pediatric resuscitation) and amiodarone (53%). Other medications present on code carts included, magnesium (43%), and procainamide (33%), bretylium, (13%) antibiotics, (10%) and potassium chloride (6%). When the data was compared between the two hospital types, medications that were more likely to be present on HIH code carts than FSH were amiodarone (71% vs. 50 %), dobutamine (86% vs. 58%) and MgSO4 (71% vs. 38 %). Over half of the HIH carried flumazenil (57%), Vasopressin (57%) and heparin (71%). There was no significant difference in any of the individual medications listed.
Conclusions: While most hospitals include medications recommended by the AHA on their code carts, not all such medications are universally present. Local practice seems to dictate the inclusion of drugs not on the recommended list, yet it would be expected that all hospitals would be consistent and current with AHA recommendations for basic resuscitation medications. Of note, the practice of including concentrated potassium vials on code carts may be particularly hazardous and against JCAHO recommendations.
Reference: JAOA Vol 104 Num 1 Jan 2004
Pediatric Code Blue Review Comparisons Between
Free-Standing Hospitals and Hospital-within-Hospital Settings.
R Berens, K Colpaert, S Percy Jr, M Quasney, R Brilli, B McGarr, TB Rice and
the NACHRI PICU FOCUS Group.
Anesthesiology and Critical Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin; National Outcomes Center, Inc., Children’s Hospital and Health Systems, Milwaukee, WI; Division of Pediatric Critical Care, Hackensack University Medical Center, Hackensack, N.J.; Le Bonheur Children’s Medical Center, Memphis, TN; Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH; St. Joseph’s Children’s Hospital of Tampa, Tampa, FL; Critical Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI
Introduction: Pediatric cardiopulmonary arrests (code blue) are
relatively rare events. JCAHO and other regulatory agencies require that
hospitals review and assess their response to cardiopulmonary arrest events.
Resources and personnel are designated to provide direct clinical care during
an arrest and then to subsequently collect data and analyze the effectiveness
of the care provided. The AHA has also established essential data elements that
they believe need to be reviewed for these events. The purpose is to
determine if the QI review process of code blue events differ between
freestanding pediatric hospitals (FSH) or pediatric facilities operating as a
hospital-within-hospital (HIH).
Methods: In 1996, the American Heart Association established a list
of practice guidelines that have been defined as both “essential” and “gold
standard” as it relates to adult cardiac arrest. A NACHRI PICU FOCUS Group Codes subcommittee surveyed and
analyzed the code blue QI review forms from NACHRI member hospitals. The
hospitals submitting the QI forms were divided by hospital type, FSH or HIH.
Questions on the QI forms were independently analyzed by two investigators (RJB
and KDC). The questions were grouped into categories of similar content
(“categories of interest”) and compared between the two hospital groups. The
questions asked by greater than 50% of the hospitals and the AHA “essential”
questions were analyzed separately between the two groups. A t-test was utilized for comparison
with significance defined as p-value<0.05.
Results: 114 NACHRI member hospitals were surveyed. 84/114(74%) of
the distributed surveys were returned. 30/84 (26%) of those hospitals returned
code blue QI review forms. 21 of the 30 hospitals were FSH and 9 were HIH. In aggregate, the QI review forms from
the 30 hospitals contained 283 questions in 20 different categories. There was
no significant difference in the total number of questions per survey between
these two groups. Analysis of categorical data indicated significant
differences in 4 categories. Among
the most frequently asked questions, there was no difference between the hospital
groups. There was no difference in the AHA “essential” questions reviewed (26
vs. 28%, p = ns) with slightly greater than 25% of the essential AHA questions
queried by either hospital type.
Most hospitals agree on who arrested; where the arrest occurred; date
and time of arrest; did the code team arrive promptly; and did the patient
survive the event.
Conclusions: The categories of interest in the review process based on the
QI forms for pediatric code blue events are similar between the two types of
hospitals, however, there is much variability regarding the specific questions
utilized within each category.
There is minimal use of the AHA guidelines for CPR QI review.
Code Team Composition in NACHRI Hospitals
S Percy Jr, K Colpaert, M Quasney, R Brilli, R Gibson, L Easterling, TB Rice, R Berens and the National Association of Children’s Hospitals and Related Institutions (NACHRI) PICU FOCUS Group
Division of Pediatric Critical Care, Hackensack University Medical Center, Hackensack, NJ; National Outcomes Center, Inc. Children’s Hospital and Health Systems, Milwaukee, WI; Le Bonheur Children’s Medical Center, Memphis, TN; Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH; Cincinnati Children’s Hospital and Medical Center, Cincinnati, OH; Cook Children’s Medical Center, Fort Worth, TX; Pediatric Critical Care Division, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI; Anesthesiology and Critical Care, Medical College of Wisconsin, Children’s Hospital of Wisconsin, Wauwatosa, WI
Introduction: Pediatric cardiopulmonary arrests (“code blue”) are rare events. One might conclude that a designated, experienced team would be identified to respond to these events in an effort to optimize patient outcome. Little information exists about the composition of these teams and how that relates to outcome. A request of NACHRI hospitals was conducted to obtain information about overall code team composition and to determine whether code team composition differs between pediatric institutions.
Methods: A NACHRI PICU FOCUS Group subcommittee surveyed NACHRI member hospitals requesting information on individuals required to respond to “code blue” events. Responding institutions were then categorized as either a free-standing pediatric facility (FSH) or a pediatric hospital-within-a-hospital (HIH). Data was then analyzed and compared using Fisher’s Exact Test based on whether a discipline was required to attend “code blue” events.
Results: 114 NACHRI hospitals were surveyed regarding which disciplines were required to attend pediatric “code blue” events. 68/84 (60%) of hospitals responded to questions about code team composition. Twenty “personnel” types representing various disciplines were identified on the survey with the option to also include individuals not identified under “Other.” Responding institutions were nearly evenly divided between the FSH (29) and HIH (31). In FSH, fellows were more likely to attend a code (p= 0.0237) as was security (p= 0.0057). The presence of a pharmacist approached but did not reach statistical significance (p=0.0695). No other discipline reached statistical significance including amongst others, anesthesiologists, PICU attendings, residents and critical care nurses.
Conclusions: Many different disciplines are represented on pediatric code teams. With the exception that fellows and security are more likely to be present at resuscitations in freestanding pediatric hospitals when compared to pediatric hospital-within-a-hospital there were no significant differences existing between the two organizational types of institutions. Further investigation is needed to determine if code team composition affects outcome.
Using Telemedicine to Provide Support During
Pediatric Resuscitations
AA Kon MD, CM and JP Marcin MD MPH
Department of Pediatrics, UC Davis Children’s Hospital, Sacramento, CA
Introduction: Many Pediatric Intensive Care Unit (PICU) physicians receive urgent telephone consultation requests for real-time pediatric resuscitation assistance. These requests may originate from Emergency Departments or hospital inpatient care areas. Recently, we have developed an innovative pediatric critical care telemedicine program that connects our PICU and the homes of our PICU attendings to rural Emergency Departments and remote inpatient care areas.
Methods: We report three cases of
pediatric resuscitations taking place at remote locations lead by a PICU
attending physician from our PICU and from home. Resuscitations were
supported by PICU attending over telemedicine to a rural Emergency Department,
to a remote hospital’s inpatient care area, and to our PICU when the attending
physician was at home.
Results: Post-resuscitation interviews
demonstrated a high degree of satisfaction among the remote patient care
team. Further, resuscitations
supported with live telemedicine consultations were ranked among remote
providers as having provided superior quality of care compared to
resuscitations not having telemedicine support.
Conclusions: Telemedicine consultation may be
superior to telephone consultation for pediatric resuscitation.
Institutions that consult with rural hospitals or are unable to provide
in-house attending coverage for their PICU may consider the use of this
technology to improve outcomes and satisfaction.
A New
Challenge in Pediatric Obesity:
Pediatric
Hyperglycemic Hyperosmolar Syndrome
RM Carchman MD,
M Dechert-Zeger MD,
AS Calikoglu MD,
BD Harris MD
From the Departments of Pediatric Critical Care (RMC, BDH) and Pediatric Endocrinology (MDZ, ASC) at the University of North Carolina Hospital at Chapel Hill, Chapel Hill, NC
Introduction: The prevalence of type 2 diabetes (T2DM) has increased in children and adolescents more than 10-fold in the last decade, and can be attributed to the epidemic of childhood obesity in the United States. One consequence of T2DM is the hyperglycemic, hyperosmolar syndrome (HHS) which can lead to multisystem organ failure or death. The mortality rate in pediatric patients is unknown, but recent case reports demonstrate extremely poor outcomes. Early recognition and adequate treatment of HHS may help decrease the mortality rate in pediatric patients.
Methods: Case report.
Results: Two of the four patients died. The first patient died within the first 24 hours of HHS presumably due to hypovolemic shock. The second patient, who died, developed rhabdomyolysis and multisystem organ failure after a prolonged ICU stay. The third and fourth patients were discharged from the hospital in good health. None of the patients had cerebral edema on head CT, despite differences in fluid and insulin management.
Conclusions: Pediatric patients with HHS have a high mortality rate and may experience multiple complications such as rhabdomyolysis and hypovolemic shock. Treatment strategies to reduce mortality are unclear and warrant further investigation.
True-True, Unrelated: A Case Report
DD Hsing MD, A Madikians MD
Pediatric Critical Care, Department of Pediatrics
Mattel Children’s Hospital at the University of California, Los Angeles; Los
Angeles, California
Introduction: Sudden cardiac deaths in previously healthy children are frequently due to undiagnosed cardiovascular diseases, either congenital or acquired. In an uncommon clinical entity known as commotio cordis, sudden death from cardiac arrest can occur in young athletes after a blunt blow to the chest, in the absence of pre-existing cardiovascular disease. We present a case in which the clinician’s high index of suspicion lead to the diagnosis of acute myocarditis in a patient whose sudden cardiac deterioration was initially attributed to late onset commotio cordis.
Methods: A case report and review of literature via Medline (1996-2004) search using the key words “myocarditis” and “commotio cordis.”
Results: A 12-year-old boy was admitted with elevated cardiac enzymes and respiratory distress after being hit in the chest with a dodge ball. Shortly after admission, patient developed refractory ventricular arrhythmia, which was thought to be the result of blunt chest trauma. Further evaluation with endomyocardial biopsy, however, demonstrated acute myocarditis as the true etiology, for which patient received immunosuppressive treatment. Unfortunately, the patient eventually required cardiac transplantation because of progressive, irreversible cardiac dysfunction due to myocarditis.
Conclusions: Although acute myocarditis and commotio cordis can present in a similar fashion, i.e. malignant ventricular arrhythmia, approaches to management of each disease are very different. This case illustrates the importance of having a broad differential diagnosis in mind when presented with a previously healthy child in sudden cardiogenic shock.
A Case Study: Management Implications of SCIWORA: CT vs. MRI
J Cabarlo J, AL Graciano, and RJ Dimand
Children’s Hospital Central California, University of San Francisco-Fresno,
Madera, California
Introduction: Diagnosis of cervical spine injury is a challenge in pediatric trauma, frequently not manifest on standard x-rays of the spine. SCIWORA Syndrome, Spinal Cord Injury Without Radiological Abnormality, based on ligamentous injury often with instability of the spine has been well described in the CT scan era. The optimal approach to the child at risk for significant c-spine injury is still evolving. Most cases involved initial evaluation with x-ray and CT scan and/or flexion/extension views. The role of MRI in the evaluation of these patients has not been thoroughly studied. In this case, a MRI demonstrated extensive ligamentous injury and spinal cord contusion in the C5 – T2 area in a patient who had persistent neurological signs and symptoms. In this case study, an argument is made for MRI should be done in the initial evaluation of high-risk patients.
Methods: A five-month-old male sustained a head and neck injury after a high-speed motor vehicle accident. The patient was sitting in his car seat restrained. By history, he was not ejected from the motor vehicle. The patient’s neck was properly immobilized. Upon initial physical exam, the patient was noted to have decreased lower extremity sensorium and movement. The initial evaluation included plain films and a head and neck CT (without contrast).
Results: No
significant abnormality noted on his neck CT. The head CT did reveal a clinically insignificant, small
subarachnoid hemorrhage. Due to
ongoing concerns based on clinical symptoms, an MRI of the spine was performed
which demonstrated significant ligamentous injury and significant contusion of
the spinal cord between C5 to T2. During his hospital admission, there were
some subtle improvements in lower extremity function, but deficits persist.
Conclusions: In the setting of head and neck trauma, a high suspicion of ligamentous injury with spine instability should be considered in the differential. This case demonstrates that CT based imaging of the neck may not be adequate for defining these injuries and MRI may be the test of choice. The original SCIWORA cases differentiated plain x-rays from CT and/or flexion/extension views, all still x-ray based methods. The term SCIWORA may no longer be applicable, as advanced radiologic imaging modalities (CT) may still have a role, while MRI (magnetic not radiographic) may evolve into the definitive exam of choice. Further studies are needed to delineate the relative value of each imaging modality in patients at high risk for ligamentous damage, spine instability and the resulting risk of a secondary cord injury. This case helps demonstrate that MRI is the diagnostic test of choice for evaluation of the high-risk patient for spinal cord injury.
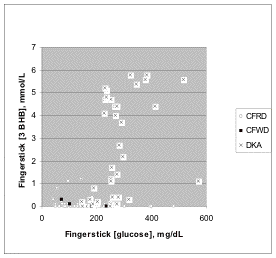 Results:
Results: