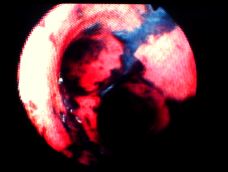
 The diagnosis of inhalation injury remains difficult because the components of the smoke to which any individual patient has been exposed are generally unknown. As current treatment is exclusively supportive, diagnosis provides only prognostic information. Bronchoscopy frequently reveals carbonaceous endobronchial debris and mucosal pallor, erythema or ulceration (Figure). But findings at the time of initial evaluation frequently only loosely correlate with the subsequent clinical course and long term sequelae.
The diagnosis of inhalation injury remains difficult because the components of the smoke to which any individual patient has been exposed are generally unknown. As current treatment is exclusively supportive, diagnosis provides only prognostic information. Bronchoscopy frequently reveals carbonaceous endobronchial debris and mucosal pallor, erythema or ulceration (Figure). But findings at the time of initial evaluation frequently only loosely correlate with the subsequent clinical course and long term sequelae.
The earliest consequence of inhalation injury is upper airway edema which is commonly seen during the first 6 to 24 hours after injury. It is common in patients with large surface area injuries who develop more significant loss of capillary integrity, resulting in soft tissue edema. Children with very severe injuries will slough casts of endobronchial debris that can cause small and large airway obstruction and may occasionally occlude the endotracheal tube. Steam inhalation will cause direct injury to the distal tracheobronchial tree, but most distal airway injuries are caused by aerosolized toxins (from burning plastics) rather than direct heat, as the upper airway is an effective heat sink.
Early obstruction of the upper airway is managed with intubation. In equivocal cases, bronchoscopy is performed and patients with significant upper airway edema are intubated using the bronchoscope as a stylet. Children can generally be extubated (if mechanics and mental status and gas exchange are satisfactory) when facial edema has largely resolved and there is an audible airleak around the endotracheal tube at a moderate pressure (20-30 cm H2O).
The later physiologic consequences of small airway obstruction and intrapulmonary shunting become more prominent after several days. Children with more serious injuries will develop progressive respiratory failure. Endobronchial debris and the loss of the ciliary clearance mechanism result in a high rate of pneumonia, which occurs in 20% to 50% of patients who suffer inhalation injury.
Management of patients with inhalation injury often requires aggressive pulmonary toilet, aided by frequent chest physiotherapy and toilet bronchoscopy. Positive pressure ventilation facilitates management of patients with large intrapulmonary shunts or mechanics poor enough to result in an excessive work of breathing. Although moderate inflating pressures will help to expand recruitable segments, peak inspiratory pressures in excess of 35-40 cm H2O should be avoided because they are associated with both overt barotrauma as well as more subtle overpressure injuries (“volutrauma”) to the pulmonary microvasculature and alveoli which can themselves exacerbate respiratory failure. High inflating pressures are also ineffective in recruiting additional lung, because the compliance decrements are not homogeneous and high pressures simply overdistend more compliant segments. Setting optimal levels of positive end-expiratory pressure may be effective in alveolar recruitment. When ventilating children with poor compliance we use pressure control ventilation and tolerate moderate hypercapnia and respiratory acidosis to facilitate control of inflating pressures . Neither steroids nor prophylactic antibiotics are routinely recommended. We rarely use early tracheostomy, and only then in those very exceptional circumstances when it is required for pulmonary toilet in those children who are sloughing large amounts of endobronchial debris.
Hyperbaric oxygen therapy may be indicated in selected children with documented or suspected clinically significant exposure to carbon monoxide (CO) to hasten clearance of carboxyhemoglobin (COHb), thus limiting ischemia secondary to a physiologic anemia, and to hasten clearance of CO bound to cytochrome enzymes, presumably limiting cellular injury. There is an extensive favorable uncontrolled literature, but major controversy persists over who should be treated. Many believe that stable patients with clear neurologic dysfunction and elevated COHb should be treated if it can be safely done, considering risks associated with transport and compromised monitoring during treatment. Children with more subtle exposures present more difficult decisions, acknowledging that obtundation after extrication from structural fires can be multifactorial in origin, secondary to anoxia, cyanide, CO, hypotension, and drugs.
Outcome for most children is very good if secondary lung injury from high inflating pressures, high concentrations of oxygen, or the adverse pulmonary sequelae of wound sepsis are avoided. Most will have clinically normal lung function, although there are subtle restrictive and obstructive changes reported with later pulmonary function studies.
A Few References
Clark WR, Jr. Smoke inhalation: diagnosis and treatment. World Journal of Surgery 16:24, 1992Boutros AR, Hoyt JL, Boyd WC, Hartford CE. Algorithm for management of pulmonary complications in burn patients. Critical Care Medicine 5:89, 1977
Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? Am J Respir Crit Care Med 150:1722, 1994
Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low volume, pressure limited ventilation with permissive hypercapnia: a prospective study. Critical Care Medicine 22:1568, 1994
Bidani A, Tzouanakis AE, Cardenas VJ, Jr., Zwischenberger JB. Permissive hypercapnia in acute respiratory failure. JAMA 272:957, 1994
Bigatello LM, Hurford WE, Kacmarek RM, Roberts JD, Jr., Zapol WM. Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome. Effects on pulmonary hemodynamics and oxygenation. Anesthesiology 80:761, 1994
Masanes MJ, Legendre C, Lioret N, Maillard D, Saizy R, Lebeau B. Fiberoptic bronchoscopy for the early diagnosis of subglottal inhalation injury: comparative value in the assessment of prognosis. Journal of Trauma 36:59, 1994
Sheridan RL, Kacmarek RM, McEttrick MM, Weber JM, Ryan CM, Doody DP, Ryan DP, Schnitzer JJ, Tompkins RG. Permissive hypercapnia as a ventilatory strategy in burned children: effect on barotrauma, pneumonia, and mortality. Journal of Trauma 39:854, 1995
Desai MH, Mlcak RP, Robinson E, McCauley RL, Carp SS, Robson MC, Herndon DN. Does inhalation injury limit exercise endurance in children convalescing from thermal injury J Burn Care Rehabil 14:12, 1993
Grube BJ, Marvin JA, Heimbach DM. Therapeutic hyperbaric oxygen: help or hindrance in burn patients with carbon monoxide poisoning? J Burn Care Rehabil. 9:249 52, 1988
Weiss LD, Van Meter KW. The applications of hyperbaric oxygen therapy in emergency medicine. Am J Emerg Med 10:558 68 1992